
Pulmonary laser-assisted metastasectomy is associated with prolonged survival in patients with colorectal cancer
Introduction
About half of the patients with colorectal cancer develop distant metastases over time, with the liver being the most common site, followed by the lungs. Despite rapid progress being made regarding the effectiveness of chemotherapy and immune therapy, complete surgical resection still remains the only hope for long-term survival for these patients (1). Numerous retrospective analyses have been conducted since the hallmark study of the International Registry of Lung Metastases, which demonstrated that pulmonary metastasectomy can potentially be a curative treatment (2). To date pulmonary metastasectomy for colorectal cancer is performed with curative intent in many institutions, yet clinical practice varies widely due to the lack of randomized data (3). According to a meta-analysis published in 2013, the 5-year survival for patients with completely resected pulmonary metastases from colorectal cancer ranges from 27–68% (4). Several factors have been found to associate with prolonged disease-free survival, such as primary tumor histopathology, the number, size and site of metastases as well as a long progression-free interval between successful treatment of the primary cancer and pulmonary metastasectomy (4). However, randomized controlled trials that help to select the patients who are most likely to benefit from surgery are still lacking.
About half of the patients presented with a history of curatively treated liver metastases. So far, there have been few studies analyzing the outcome of patients after metastasectomy of both liver and pulmonary metastases originating from colorectal cancer. Long-term disease-specific survival of more than 10 years has been described in a small number of patients, making a generally more aggressive surgical management of metastases an option for selected patients, especially since morbidity and mortality are very low in experienced centers (5).
Laser-assisted pulmonary metastasectomy is an increasingly popular, safe and lung parenchyma saving technique (6-9). More metastases can potentially be resected per patient and a better local control has also been reported (7,9). The aim of this study was to evaluate if the technique of laser-assisted surgery (LAS) makes pulmonary metastasectomy a more accessible option for selected patients with colorectal cancer and to analyze the impact a history of curatively treated liver metastases might have.
Methods
Patients
We performed a retrospective analysis of the database of medical records at the Department of Thoracic Surgery, Medical Center – University of Freiburg, a tertiary care cancer center. A total of 204 patients were identified (135 males and 69 females) who underwent pulmonary metastasectomy for colorectal cancer with curative intent from 01/2005 to 12/2016. All patients underwent surgical therapy of the primary tumor. Patients presenting with metastases to other organs except curatively treated liver metastases were excluded, as well as patients with malignant pleural effusion or a known second malignancy at the time of metastasectomy.
Prior to metastasectomy all patients underwent analysis of the pulmonary function to be compatible with the intended procedure. Anterolateral thoracotomy or video-assisted thoracoscopy (VATS) were used for the resection of the metastases. Some patients underwent more than one surgery for each side in case of recurrent metastases. Staged thoracotomy was done in case of bilateral metastases. LAS was performed using a Nd:YAG (neodymium-doped yttrium aluminum garnet; Nd:Y3Al5O12) laser (1,320 nm; LIMAX, KLS Martin GmbH & Co. KG, Tuttlingen, Germany).
Definitions
Data were provided by the registry of the CCCF (Comprehensive Cancer Center Freiburg) and obtained by going through hospital records. We separated disease-specific death from death related to other causes, when clearly stated in the records. Sometimes the line between disease-related death and death related to other causes is blurred and a decision for one group or the other always implies a certain bias, no matter how carefully it is done. Survival was defined as the interval between pulmonary metastasectomy and the last follow-up or event. Resection status was defined as R0 if the metastases were completely removed, R1 in case of incomplete removal and categorized as R2 in case patients decided against contralateral surgery.
The extent of mediastinal lymphadenectomy concurrent with metastasectomy varies greatly since it was the individual decision of the surgeon. Chemotherapy within 8 weeks before or after metastasectomy was counted as adjuvant treatment. Perioperative complications were defined as complications arising within 30 days of pulmonary metastasectomy. All patients underwent regular clinical follow-ups, including computed tomography of the chest and abdomen as well as blood tests. Individual consent for the study was obtained from all patients. The study was approved by the Medical Center – University of Freiburg’s local ethics committee and it is registered at the German Registry for Clinical Trials under the trial registration number 00011900.
Statistical analysis
Survival was estimated using the Kaplan-Meier method (10). For comparison of survival curves the log rank test was used. Multivariate analysis using the Cox proportional hazards regression model was carried out after prognostic factors were identified in univariate analysis. Mann-Whitney test was used as appropriate. The threshold level for statistical significance for all analyses was a P value <0.05. All statistical analysis was conducted using GraphPadPrism (Version 7, GraphPad Software Inc., La Jolla, CA, USA) and SPSS software (Version 23, IBM Corporation, New York, NY, USA).
Results
We included 204 patients (135 males and 69 females) who underwent pulmonary metastasectomy for colorectal cancer with curative intent at our institution from January 2005 to December 2016 (Table 1). Overall 5-year disease-specific survival was 62%. Seventy-seven (38%) patients underwent LAS while in 127 (62%) cases other techniques were used. One hundred and thirteen (55%) patients were alive at the time of analysis, 65 (51%) in the NLAS group and 48 (62%) in the LAS group (Figure 1). The median follow-up was 53 months (95% CI: 47–60 months). R0-resection was achieved in 123 (97%) patients of the NLAS group and in 71 (92%) patients in the LAS group. There was a median interval of 32 months (range, −4 months to 136 months) in the NLAS group and 19 months (range, 1–136 months) in the LAS group between surgery of the primary tumor and pulmonary metastasectomy (P=0.008). A total of 267 metastases were resected in 154 operations in the NLAS group (median: 1), compared to a total of 438 metastases in 122 operations in the LAS group (median: 5; P<0.0001) (Figure 2). The median age at the time of surgery for colorectal cancer was 62 years (range, 34–84 years) in the NLAS group and 60 years (range, 32–77 years) in the LAS group, the median age at the time of pulmonary metastasectomy was 66 years (range, 39–88 years) in the NLAS group and 63 years (range, 33–81 years) in the LAS group. Seventeen (22%) patients in the LAS group and 13 (10%) in the NLAS group underwent adjuvant chemotherapy. Despite more negative predictors in the LAS group, overall disease-specific 5-year survival was 70% in the LAS group vs. 58% in the NLAS group (P=0.18) (Table 2).
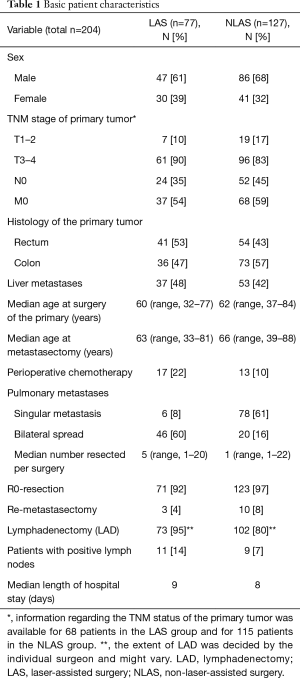
Full table
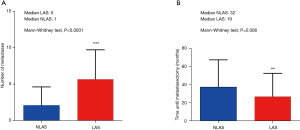
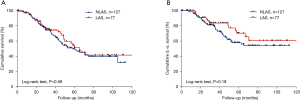
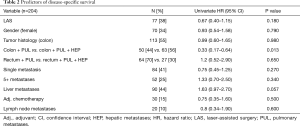
Full table
All patients in the LAS group and the majority of the patients in the NLAS group (81%) underwent thoracotomy. Three (4%) patients in the LAS group and 10 (8%) patients in the NLAS group respectively underwent re-thoracotomy for recurrence of pulmonary metastases. Staged thoracotomy for bilateral metastasis was performed in 46 (60%) patients in the LAS group and in 20 (16%) in the NLAS group. Anatomical resections were performed in 29 (23%) patients in the NLAS group and in only 6 (8%) cases in the LAS group. Overall perioperative mortality was 0%. There was a similar pattern of minor complications associated with surgery with the exception of pneumonia, which in our cohort occurred in 8% of the patients in the LAS group, compared to 3% in the NLAS group (Table 3). Concurrent mediastinal lymphadenectomy was performed to a varying degree in the majority of patients. As previously shown in several studies, independent of the surgical method used for metastasectomy, we found the presence of lymph node metastases to associate with decreased disease-specific 5-year survival (35% vs. 71%; P=0.001).
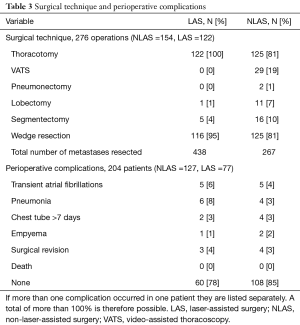
Full table
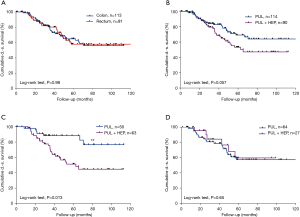
Patients with metastases originating from either rectal or colonic cancer were equally distributed in both groups. Overall disease-specific survival was not dependent on sex (P=0.79) or the histology of the primary tumor (P=0.98). Thirty-seven (48%) patients in the LAS group and 53 (42%) in the NLAS group had a history of curatively treated liver metastases. There was no statistically significant difference in overall disease-specific survival in both groups (P=0.057). However, looking specifically at patients with pulmonary metastases originating from colonic cancer, we found they had a 5-year disease-specific survival of 88% compared to the respective patients with an additional history of liver metastases (5-year disease-specific survival 51%; P=0.013). There was no significant difference in overall disease-specific survival in patients with pulmonary metastases originating from rectal cancer with vs. without a history of liver metastases (5-year disease-specific survival was 57% vs. 60%; P=0.65) (Figure 3).
Discussion
Pulmonary metastasectomy is an established treatment option with low morbidity and mortality for patients with colorectal cancer and limited liver and/or pulmonary metastases and can potentially lead to a prolonged disease specific survival (4,5,11).
The major limitation of this study is that there might be selection bias due to its retrospective nature and the overall comparatively low number of patients, especially if subgroups are formed. However, some limited statistical analysis could be done.
The majority of patients underwent thoracotomy in order to bimanually palpate the lungs for nodules that were not detectable in the chest CT scan. A minimally invasive approach was chosen for only 29 (19%) lesions in case of a solitary metastasis in the periphery of the lung. Equal survival could previously be shown after VATS surgery compared to thoracotomy in several cases (12), yet extent and technique of surgery required for detecting all nodules remain avidly discussed (12-16). A combined approach using a minithoracotomy that allows thorough palpation of the lung and performing the resections under video-assisted thoracoscopic control has also been proposed (17). However randomized controlled trials are still lacking.
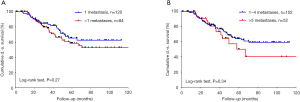
More patients in the LAS group presented with bilateral spread of metastases compared to the NLAS group, which is usually associated with reduced overall disease-specific survival (18). Interestingly, there was no statistically significant advantage in survival for patients with only a singular lung metastasis in our cohort (Figure S1).
The laser allows a very precise dissection and coagulation of the lung tissue surrounding the lung metastases and functional lung parenchyma can be preserved to the greatest possible extent (17), however sometimes an anatomical resection cannot be avoided. It could be shown that the laser causes significantly less tissue damage compared to the monopolar cutter (19) Hence, a major benefit of the tissue-saving LAS technique is that the need for anatomical resections was significantly reduced and, it is possible to be less restrictive when it comes to surgical removal of bilateral bigger and more centrally located pulmonary metastases, provided the lung function of the patient allows it (20,21). All of the resected tumors in the LAS group were sent to pathology for examination, however, for the few very small ones that were merely evaporated we had to assume that the tumor was also a metastasis. Evaporation was always done with a sufficient margin to make sure the nodule was macroscopically completely removed. Naturally, the possibility of remaining tumor cells, although highly unlikely, could not be fully excluded.
Our cohort contained an about equal number of patients with metastases arising from rectal cancer as well as colonic cancer. Though in most cases they are referred to as colorectal cancer, the two entities are, in fact, quite different (22).
The initial standard treatment options differ and the pattern of metastases has been shown to be predominantly abdominal in case of colonic cancer (liver) and tends to be more extra-abdominal in case of rectal cancer (lung and bone) (23). In our study, there was no statistically significant difference in overall disease-specific survival for patients with metastases originating from rectal compared to colonic cancer. However, we found patients with colonic cancer and pulmonary metastases but without a history of liver (or any other) metastases, to have an exceptionally good 5-year survival rate of 88% compared to 51% for patients presenting with a history of liver metastases in our study. Interestingly, the presence of additional liver metastases caused no difference in disease-specific survival for patients with metastases originating from rectal cancer. Metastatic spread to more than one organ often results in reluctance regarding metastasectomy since it may very well represent disseminated disease (5). However, our data support a role of surgery for selected patients in the setting of a multimodal oligometastatic treatment model, as long as there is a local treatment option with curative intent for each site.
In total, 22% of the patients in the LAS group and 10% in the NLAS group underwent chemotherapy within 8 weeks before or after the resection of pulmonary metastases. While definite guidelines are still lacking, it could previously be shown that about 75% of the patients relapse after metastasectomy and that adjuvant chemotherapy can potentially prolong the disease-free interval (1,24). There was no statistically significant difference in overall disease-specific survival for patients with and without chemotherapy in our cohort, however, due to the small number of patients and slightly varying therapy regimes and time points, it is not possible to come to a conclusion regarding the benefit of adjuvant systemic treatment in this context. To further elucidate the role of chemotherapy in the context of pulmonary metastasectomy, prospective randomized trials are needed.
It could be demonstrated in numerous analyses that positive lymphatic nodes are associated with a decrease in long-term survival (3,4,18,25). In out small cohort, the reduction in disease-specific survival was not statistically significant for patients with positive lymph nodes, but again, due to the small number of patients and the varying individual surgical approach a valid conclusion regarding its benefit cannot be made based on our data. In fact, in many European institutions the extent of lymph node removal has not been standardized yet (3), whereas the benefit of an assessment of the lymphatic nodes in order to evaluate the indication for additional chemotherapy has been widely acknowledged (26).
Long-term disease-specific survival has previously been shown to be associated with a longer progression-free interval and a singular metastasis in numerous studies (6,18,27).
We found in our retrospective analysis that, provided complete resectability, long-term disease-specific survival is possible for selected patients with a larger number of metastases, bilateral spread and overall faster progressing tumors, as indicated by a shorter diseases-free interval. This also seems to hold true for patients with a history of curatively treated liver metastases.
Thus we conclude that laser-assisted resection allows complete removal of a larger number of bilateral and even centrally located pulmonary metastases with relatively few side effects, considered the lung function and overall condition of the patient allow it. Overall disease-specific survival appears to be comparable to patients with less and later occurring metastases, making this an option available to a broader range of patients.
Larger and possibly prospective trials are needed to further corroborate those findings and further define the role of pulmonary metastasectomy in the multimodal therapy.
Acknowledgments
The authors thank Mrs. Maja von Cube for expert statistical assistance. Part of this work was used for Friederike Funcke’s MD thesis (Dr. med.).
Footnote
Conflicts of Interest: Meeting presentations: Part of this work was presented at the annual meeting of the German Thoracic Surgery Society (DGT), 10/19/17–10/20/17 in Munich, Germany.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Medical Center – University of Freiburg’s local ethics committee and it is registered at the German Registry for Clinical Trials under the trial registration number 00011900.
References
- Brandi G, Derenzini E, Falcone A, et al. Adjuvant systemic chemotherapy after putative curative resection of colorectal liver and lung metastases. Clin Colorectal Cancer 2013;12:188-94. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Internullo E, Cassivi SD, Van Raemdonck D, et al. Pulmonary metastasectomy: a survey of current practice amongst members of the European Society of Thoracic Surgeons. J Thorac Oncol 2008;3:1257-66. [Crossref] [PubMed]
- Gonzalez M, Poncet A, Combescure C, et al. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 2013;20:572-9. [Crossref] [PubMed]
- Neeff H, Horth W, Makowiec F, et al. Outcome after resection of hepatic and pulmonary metastases of colorectal cancer. J Gastrointest Surg 2009;13:1813-20. [Crossref] [PubMed]
- Rolle A, Pereszlenyi A, Koch R, et al. Is surgery for multiple lung metastases reasonable? A total of 328 consecutive patients with multiple-laser metastasectomies with a new 1318-nm Nd:YAG laser. J Thorac Cardiovasc Surg 2006;131:1236-42. [Crossref] [PubMed]
- Franzke K, Natanov R, Zinne N, et al. Pulmonary metastasectomy - A retrospective comparison of surgical outcomes after laser-assisted and conventional resection. Eur J Surg Oncol 2017;43:1357-64. [Crossref] [PubMed]
- Macherey S, Doerr F, Wahlers T, et al. Pneumologie 2017;71:475-9. [Role of Laser Resection in Pulmonary Metastasectomy]. [Crossref] [PubMed]
- Osei-Agyemang T, Palade E, Haderthauer J, et al. Zentralbl Chir 2013;138 Suppl 1:S45-51. [Pulmonary metastasectomy: an analysis of technical and oncological outcomes in 301 patients with a focus on laser resection]. [Crossref] [PubMed]
- Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc 1958;53:457-81. [Crossref]
- Meimarakis G, Spelsberg F, Angele M, et al. Resection of pulmonary metastases from colon and rectal cancer: factors to predict survival differ regarding to the origin of the primary tumor. Ann Surg Oncol 2014;21:2563-72. [Crossref] [PubMed]
- Mutsaerts EL, Zoetmulder FA, Meijer S, et al. Long term survival of thoracoscopic metastasectomy vs metastasectomy by thoracotomy in patients with a solitary pulmonary lesion. Eur J Surg Oncol 2002;28:864-8. [Crossref] [PubMed]
- Pfannschmidt J, Egerer G, Bischof M, et al. Surgical intervention for pulmonary metastases. Dtsch Arztebl Int 2012;109:645-51. [PubMed]
- Yano T, Shoji F, Maehara Y. Current status of pulmonary metastasectomy from primary epithelial tumors. Surg Today 2009;39:91-7. [Crossref] [PubMed]
- Shiono S, Okumura T, Boku N, et al. Outcomes of segmentectomy and wedge resection for pulmonary metastases from colorectal cancer. Eur J Cardiothorac Surg 2017;51:504-10. [PubMed]
- Eckardt J, Licht PB. Thoracoscopic or open surgery for pulmonary metastasectomy: an observer blinded study. Ann Thorac Surg 2014;98:466-9; discussion 469-70. [Crossref] [PubMed]
- Meyer C, Bartsch D, Mirow N, et al. Video-Assisted Laser Resection of Lung Metastases-Feasibility of a New Surgical Technique. Thorac Cardiovasc Surg 2017;65:382-6. [Crossref] [PubMed]
- Hornbech K, Ravn J, Steinbruchel DA. Current status of pulmonary metastasectomy. Eur J Cardiothorac Surg 2011;39:955-62. [Crossref] [PubMed]
- Kirschbaum A, Braun S, Rexin P, et al. Comparison of local tissue damage: monopolar cutter versus Nd:YAG laser for lung parenchyma resection. An experimental study. Interact Cardiovasc Thorac Surg 2014;18:1-6. [Crossref] [PubMed]
- Welter S, Cheufou D, Ketscher C, et al. Risk factors for impaired lung function after pulmonary metastasectomy: a prospective observational study of 117 cases. Eur J Cardiothorac Surg 2012;42:e22-7. [Crossref] [PubMed]
- Welter S, Cheufou D, Zahin M, et al. Short- and Mid-Term Changes in Lung Function after Bilateral Pulmonary Metastasectomy. Thorac Cardiovasc Surg 2016;64:139-45. [PubMed]
- Qiu M, Hu J, Yang D, et al. Pattern of distant metastases in colorectal cancer: a SEER based study. Oncotarget 2015;6:38658-66. [Crossref] [PubMed]
- Hugen N, van de Velde CJ, de Wilt JH, et al. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol 2014;25:651-7. [Crossref] [PubMed]
- Subbiah IM, Blackmon SH, Correa AM, et al. Preoperative chemotherapy prior to pulmonary metastasectomy in surgically resected primary colorectal carcinoma. Oncotarget 2014;5:6584-93. [Crossref] [PubMed]
- Bolukbas S, Sponholz S, Kudelin N, et al. Risk factors for lymph node metastases and prognosticators of survival in patients undergoing pulmonary metastasectomy for colorectal cancer. Ann Thorac Surg 2014;97:1926-32. [Crossref] [PubMed]
- Pfannschmidt J, Klode J, Muley T, et al. Nodal involvement at the time of pulmonary metastasectomy: experiences in 245 patients. Ann Thorac Surg 2006;81:448-54. [Crossref] [PubMed]
- Osoegawa A, Kometani T, Fukuyama S, et al. Prognostic Factors for Survival after Resection of Pulmonary Metastases from Colorectal Carcinoma. Ann Thorac Cardiovasc Surg 2016;22:6-11. [Crossref] [PubMed]


