The role of red blood cell distribution width in mortality and cardiovascular risk among patients with coronary artery diseases: a systematic review and meta-analysis
Brief summary
The red cell distribution width (RDW) is a quantitative measure of the size variation of circulating erythrocytes. Of note, RDW might be a novel biomarker that reflects multiple physiological impairments related to atherosclerosis and coronary artery disease. However, conflicting findings have been reported on the association of RDW with risk of subsequent cardiovascular (CV) events. We therefore conducted this systematic review and meta-analysis to synthesize all available evidence of prospective studies on this interesting issue.
Introduction
RDW is a quantitative measure of the size variation of circulating erythrocytes with higher values reflecting greater heterogeneity in cell sizes (1). RDW is routinely reported to physicians in clinical practice as part of the automated complete blood count (CBC), which mainly served as an auxiliary index in the differential diagnosis of microcytic anemia (2).
In addition to its traditionally known role, RDW is of interest for its potential impact on cardiovascular disease (CVD) and mortality risk in general population (3-5). A previous meta-analysis and prospective studies have showed that higher RDW, even within the normal reference range, was strongly associated with increased risk of death and CVD risk in community-dwelling adults (3-5). Of note, RDW significantly improved mortality risk prediction beyond established risk factors, as assessed by several indices of model calibration and discrimination. Although the exact mechanisms are unclear, this association is provocative because it is independent of numerous factors, including nutritional status, anemia, inflammation, and others co-morbidity diseases. Hence, it is possible that RDW is a novel biomarker that reflects multiple physiological impairments related to atherosclerosis and coronary artery diseases (CAD).
However, conflicting findings have been reported on the association of RDW with risk of subsequent CVD events during prospective follow-up of individuals with established CAD (3-5). With implications for drug development and secondary prevention, the pathogenesis of first and subsequent CVD events may not be precisely equivalent. We therefore conducted this systematic review and meta-analysis to synthesize all available evidence of prospective studies that report the association of RDW in relation to all-cause mortality and fatal/non-fatal CVD events in patients with prior CAD.
Methods
A prospective protocol of objectives, literature-search strategies, inclusion and exclusion criteria, outcome measurements, and methods of statistical analysis was prepared a priori according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and the Meta-analysis of Observational Studies in epidemiology (MOOSE) guideline (6,7).
Search strategy
Study data were obtained through the following ways: searching on PubMed (1948 to January 2013), EMBASE (1948 to January 2013) and Cochrane Library database included terms “red blood cell distribution width”, “mortality” and “CV events” and their corresponding index words using the special features in titles and/or abstracts. Only human studies were included. To identify further potentially relevant studies missed by the electronic database search, reference lists of identified trials and review reports were manually screened and searches are based on English language only. In addition, we verified the search strategy by hand-searching the reference lists of primary studies, review articles, and clinical guidelines. Email-alerts with newly published articles from MEDLINE were checked until October 1, 2013.
Inclusion and exclusion criteria
Inclusion criteria were the following: (I) serum or plasma RDW was the determinant; (II) outcomes: all-cause mortality, fatal and/or non-fatal CV events such as CV death, myocardial infarction (MI), stroke, heart failure (HF) and readmission; (III) paper type: original prospective quantitative cohort study (i.e., no review, commentary, case reports, editorial); (IV) study performed in participants ≥18 years. We excluded studies that did not report any of the outcomes mentioned above. The titles and abstracts of studies identified by the search strategy were independently screened by two reviewers (LZ.L. and C.S.). Differences between authors were resolved by consensus or by consultation of an additional reviewer (X.Z.W).
Quality assessment and data extraction
A quality checklist was developed to determine the quality of the eligible studies based on the PRISMA Statement and the MOOSE guideline in combination with a previously published quality checklist for observational studies (6-8) (see Table 1). Studies were scored based on nine quality criteria on a binary scale; including description of characteristics of the study population, assessment of exposure and outcome, confounding and potential flaws. The quality of each study was independently assessed by Y.S and W.Y.M and scores were compared for each item of the checklist. The scores were summed and quality was considered poor [0-4], moderate [5-6], or good [7-9]. Studies with potential flaws or rated as poor quality were not included.

Full table
Of the eligible studies, information including first author, years of follow-up, country of origin, name of the study, number of participants, participants’ characteristics, determination of outcome, RDW concentrations and category, and outcome measures were recorded. When the report did not contain sufficient details to adjudicate the validity of the study, or outcome data were missing, attempts were made to contact the authors by email and in writing.
Data synthesis and statistical methods
Results reported as count data were presented for all-cause mortality, fatal CVD events and non-fatal CVD events: adjusted hazard ratio (HR), odd ratio (OR) or regression coefficients (β) and 95% confidence interval (CI). We extracted the results of the highest versus lowest RDW concentrations and used the lowest RDW category as the reference. If the study reported more than one estimate, only the result of the largest RDW difference was included. We transformed risk estimates by taking their natural logarithms and calculated the standard errors as follows: (Ln upper limit - Ln HR)/1.96. We weighted the natural logarithm of the risk estimates by generic inverse variance to account for the sample size and distribution of the included studies (9).
We used Review Manager 5.2 (The Cochrane Collaboration, Oxford, United Kingdom) to analyze the collected data. The results of the included studies were pooled and meta-analyses were carried out using fixed or random-effects models. Statistical heterogeneity between studies was assessed using the chi-square test with significance set at P<0.10 and heterogeneity was quantified using the I2 statistic. I2 values represent the proportion of total variation attributable to heterogeneity rather than chance whereby 0% is no observed heterogeneity and 100% maximal heterogeneity.
Potential publication bias was evaluated by visual inspection of a funnel plot. A priori sensitivity analyses were defined to evaluate the stability of the pooled estimates and to examine changes in results after excluding specific studies. The subgroup analyses were preplanned for: length of follow-up >4 years, subtype of CAD [acute coronary syndrome (ACS), coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI)] and RDW as dichotomous values. The authors had full access to the data and take responsibility for its integrity. All authors have read and agreed to the manuscript as written.
Results
Search results
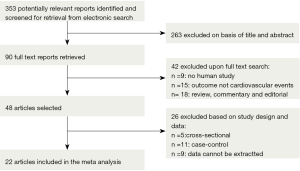
The systematic literature search yielded 353 potentially relevant articles. Figure 1 shows the flow diagram for the identification process. Briefly, 263 articles were excluded based on title and abstract. The remaining 90 studies were identified in full-text; 42 studies were excluded for study type and outcome. Then, another 26 studies were excluded based on study design (n=5 cross-sectional, n=11 case-control, n=9 data cannot be extracted). The hand search did not result in any additional articles. The email alerts yielded in two additional studies. Twenty-two studies were eligible for quality assessment and ranked according to their sum scores (see Table 1), of which 20 studies were grated as “good” and 2 as “moderate” quality. Agreement between the two reviewers was 97% for study selection and 96% for quality assessment of trials.
Study characteristics
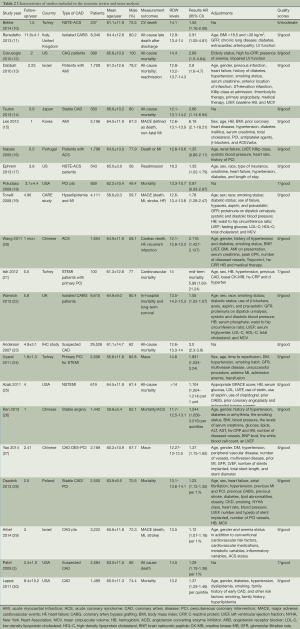
Table 2 represents the main characteristics and results of the included studies on mortality and CVD events. Of the 22 studies (10-30), 15 investigated the relation between RDW and all-cause mortality, four and eight investigated fatal and non-fatal CVD events, respectively. Six studies investigated the relation between RDW and fatal and/or non-fatal CVD events. The total number of participants was 80,216. The population sizes of the studies, all published between 2007 and 2014, varied between 100 and 29,526. The mean study duration ranged between 1 month and 23 years. Overall, 22 different cohorts were used of which one included only men (12). Ten cohorts were conducted in Asia, six in the United States and four in Europe; mean age ranged between 55.6 and 66.6 years. Risk estimates (HRs/ORs), regression coefficients, and 95% CI were reported in 15 studies for RDW levels (highest vs. lowest) or dichotomous cut-off values of RDW, which were include in the subsequent meta-analysis. Additionally, risk estimates of RDW were reported as a continuous variable in seven studies.
Full table
RDW and all-cause mortality
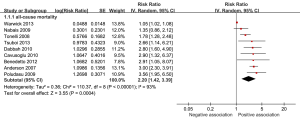
Of the 15 studies that were included in the meta-analysis, all studies reported positive associations between high RDW concentrations and all-cause mortality, except one studies reported negative association (16). In the meta-analysis, the summary estimates for the highest compared with lowest category of baseline RDW indicated a significant increased risk for all-cause mortality in CAD patients: pooled risk ratio (RR) 2.20 (95% CI, 1.42-3.39; P<0.0004) (Figure 2). Heterogeneity was relatively high present (I2 =93%).
RDW and fatal/non-fatal CVD events
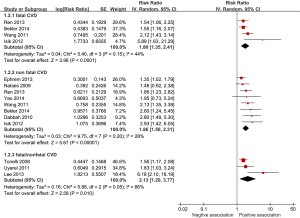
Four studies investigated excess RDW level and fatal CVD events specifically (10,20,21,26). Eight studies (10,13,16,17,20,21,26,27) investigated high RDW and non-fatal CVD events and three studies (14,15,19,24) reported the association between RDW and fatal/non-fatal CVD events. All studies reported positive associations between high RDW concentrations and fatal CVD events. For non-fatal CVD events, six studies reported positive associations and in two studies (16,17) higher RDW was not significantly associated with non-fatal CVD events. One study reported cardiac events for HF and stroke separately. In the meta-analysis, the pooled estimates for the highest compared with lowest category of baseline RDW indicated a significant increased risk for fatal CVD events: pooled RR 1.80 (95% CI, 1.35-2.41; P<0.0001), with a moderate heterogeneity (I2 =44%). The pooled estimate for non-fatal CVD events resulted in a slightly higher association: RR 1.86 (95% CI, 1.50-2.31; P<0.00001), with a low heterogeneity (I2 =28%). The pooled estimate for the overall fatal/non-fatal CVD events resulted in a higher association: RR 2.13 (95% CI, 1.20-3.77; P=0.01; I2 =66%) (see Figure 3).
Publication bias and sensitivity analysis
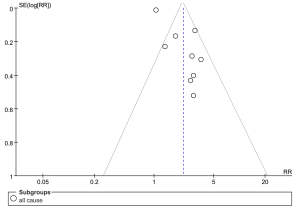
The funnel plot (Figure 4) for studies of RDW and all-cause mortality shows reasonable symmetry at the top of the funnel plot and a little asymmetry at the bottom, which suggest some evidence of publication bias for smaller studies.
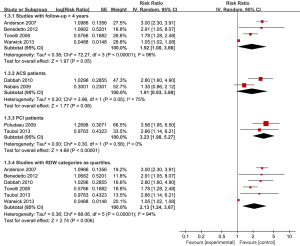
The findings were similar whether fixed or random-effects models were used. However, sensitivity analyses for follow-up duration >4 years only marginally changed the results (Figure 5). The inclusion of studies with ACS patients attenuated the results; nonetheless, the estimate was still significant: RR 1.91 (95% CI, 0.93-3.89; P=0.08; I2 =75%). The inclusion of studies with PCI patients resulted in a more pronounced estimate and negligible heterogeneity: HR 3.23 (95% CI, 1.98-5.27; P<0.0001; I2 =0%). No sensitivity analyses were performed for fatal CVD events, because of too few studies.
Discussion
Our study provides the first systematic review and meta-analysis of prospective studies of RDW and total mortality, fatal and non-fatal CVD events in populations with established CAD. The meta-analysis indicates a significant increased risk for RDW excess and all-cause mortality and CVD events that range from 80% to 120%.
Our systematic review and subsequent meta-analysis has several strengths. This first systematic review gives a broad overview of all prospective studies on RDW in relation to all-cause mortality and fatal/non-fatal CVD events, and provides insight in its associated risks. The systematic review and meta-analysis were performed according to the PRISMA Statement and the MOOSE guideline and included a quality assessment-an important component to evaluate the methodological quality (31,32). The quality assessment allowed us to distinguish between poor, moderate and good quality studies and to the selection of only moderate and good quality studies. This resulted in studies with multivariable adjusted risk estimates and a large number of included CVD cases >80,000. Moreover, the present analysis included only prospective cohort studies and most follow-ups lasted more than 23 years (30), which limits the problem of reverse causation bias.
The meta-analysis of observational studies might be influenced by heterogeneity. The variance between the included studies could partly be due to differences in study populations, RDW assays, outcome definitions, and adjustment for confounders. The use of random-effects models in our analyses adjusts in part for these variances between studies (9). Subgroup analyses were performed to test the stability of the pooled estimates. The positive association between higher RDW concentrations and CVD events was consistent given the similar pooled risk estimates for total mortality, fatal and non-fatal CVD events in both fixed and random models. This suggests that higher RDW level might be involved in pathological processes that lead to CVD. Of note, the inclusion of studies with PCI patients resulted in a higher HR, which might indicate that PCI patients are more prone to RDW excess and thereby have a higher risk of developing a secondary CVD event.
However, it should be noted that the results of our meta-analysis could not identify whether RDW is a causal factor for CVD. The studies in the meta-analysis used different approaches to define RDW categories. This might have affected our results, although we took into account the result of quartile 4 versus quartile 1 when available and used this approach consistently for all included studies. Sensitivity analyses for RDW and total mortality based on RDW quartiles resulted in a similar estimate than studies that used dichotomous cut-off values: pooled RR 2.13 (95% CI, 1.24-3.67). RDW cut-off values reflect a smaller difference in RDW concentration between RDW groups compared with RDW quartiles. However, pooling studies that reported cut-off values still showed a significant RR, which suggests that moderate elevations in RDW concentrations could play a role in the development of mortality risk of CAD patients.
Visual inspection of the funnel plot illustrates that studies with a smaller standard error at the top of the funnel plot were more symmetrically distributed than studies with a larger standard error at the bottom of the funnel plot (9). This suggests possible publication bias favoring smaller studies with significant results, which implies that the pooled estimate could be an overestimation of the true association; however, the power to detect publication bias is low given the limited number of studies. In addition, negative studies are less likely to be published and not all endpoints of the included studies were adjudicated and definitions of end points could be different between studies.
Based on the available prospective studies and the absence of randomized controlled trials, this meta-analysis highlights a risk factor for subsequent mortality and CVD events in populations with established CAD. Randomized controlled trials in these persons are therefore warranted to determine whether RDW-modifying therapies could result in less CVD events. It should be noted that lack of a gold standard to measure RDW hampers clinical decision making and treatment for subjects with high RDW concentrations. Nonetheless, more awareness should be given to individuals with a high RDW concentration in CAD populations.
Conclusions
Our meta-analysis supports that higher RDW concentrations are associated with increased risk of subsequent mortality risk and CVD events in established CAD patients. Despite the possibility of some publication bias, the results provide evidence for positive associations considering the quality, direction and magnitude of the associations of the included studies. Future studies should focus on RDW-modifying therapies to give more insight into the underlying mechanisms that might lead to CVD events.
Acknowledgements
This study was supported by National Natural Science Foundation of China (No.81102709), Technology Planning Project of Guangdong Province in China (2010B031600068 and 2011B031800137), Doctor’s Research Fund of Science Education Ministry of China (No.20110171120056). This manuscript has been read and approved by all the authors, the requirements for authorship have been met, everyone who qualifies for authorship has been listed as an author and each author believes that the manuscript represents honest work.
Disclosure: The authors declare no conflict of interest.
References
- Lippi G, Plebani M. Red blood cell distribution width (RDW) and human pathology. One size fits all. Clin Chem Lab Med 2014;52:1247-9. [PubMed]
- Viswanath D, Hegde R, Murthy V, et al. Red cell distribution width in the diagnosis of iron deficiency anemia. Indian J Pediatr 2001;68:1117-9. [PubMed]
- Patel KV, Ferrucci L, Ershler WB, et al. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch Intern Med 2009;169:515-23. [PubMed]
- Perlstein TS, Weuve J, Pfeffer MA, et al. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med 2009;169:588-94. [PubMed]
- Chen PC, Sung FC, Chien KL, et al. Red blood cell distribution width and risk of cardiovascular events and mortality in a community cohort in Taiwan. Am J Epidemiol 2010;171:214-20. [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [PubMed]
- Reeves BC, Deeks JJ, Higgins JP, et al. Chapter 13: Including non-randomized studies. In: Higgins JPT, Green S. eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1. Vol. 5. Chichester: The Cochrane Collaboration; 2008.
- Bekler A, Gazi E, Tenekecioglu E, et al. Assessment of the relationship between red cell distribution width and fragmented QRS in patients with non-ST elevated acute coronary syndrome. Med Sci Monit 2014;20:413-9. [PubMed]
- Benedetto U, Angeloni E, Melina G, et al. Red blood cell distribution width predicts mortality after coronary artery bypass grafting. Int J Cardiol 2013;165:369-71. [PubMed]
- Cavusoglu E, Chopra V, Gupta A, et al. Relation between red blood cell distribution width (RDW) and all-cause mortality at two years in an unselected population referred for coronary angiography. Int J Cardiol 2010;141:141-6. [PubMed]
- Dabbah S, Hammerman H, Markiewicz W, et al. Relation between red cell distribution width and clinical outcomes after acute myocardial infarction. Am J Cardiol 2010;105:312-7. [PubMed]
- Tsuboi S, Miyauchi K, Kasai T, et al. Impact of red blood cell distribution width on long-term mortality in diabetic patients after percutaneous coronary intervention. Circ J 2013;77:456-61. [PubMed]
- Lee JH, Yang DH, Jang SY, et al. Incremental predictive value of red cell distribution width for 12-month clinical outcome after acute myocardial infarction. Clin Cardiol 2013;36:336-41. [PubMed]
- Nabais S, Losa N, Gaspar A, et al. Association between red blood cell distribution width and outcomes at six months in patients with acute coronary syndromes. Rev Port Cardiol 2009;28:905-24. [PubMed]
- Ephrem G. Red blood cell distribution width is a predictor of readmission in cardiac patients. Clin Cardiol 2013;36:293-9. [PubMed]
- Poludasu S, Marmur JD, Weedon J, et al. Red cell distribution width (RDW) as a predictor of long-term mortality in patients undergoing percutaneous coronary intervention. Thromb Haemost 2009;102:581-7. [PubMed]
- Tonelli M, Sacks F, Arnold M, et al. Relation Between Red Blood Cell Distribution Width and Cardiovascular Event Rate in People With Coronary Disease. Circulation 2008;117:163-168. [PubMed]
- Wang YL, Hua Q, Bai CR, et al. Relationship between red cell distribution width and short-term outcomes in acute coronary syndrome in a Chinese population. Intern Med 2011;50:2941-5. [PubMed]
- Isik T, Kurt M, Ayhan E, et al. The impact of admission red cell distribution width on the development of poor myocardial perfusion after primary percutaneous intervention. Atherosclerosis 2012;224:143-9. [PubMed]
- Warwick R, Mediratta N, Shaw M, et al. Red cell distribution width and coronary artery bypass surgery. Eur J Cardiothorac Surg 2013;43:1165-9. [PubMed]
- Anderson JL, Ronnow BS, Horne BD, et al. Usefulness of a complete blood count-derived risk score to predict incident mortality in patients with suspected cardiovascular disease. Am J Cardiol 2007;99:169-74. [PubMed]
- Uyarel H, Ergelen M, Cicek G, et al. Red cell distribution width as a novel prognostic marker in patients undergoing primary angioplasty for acute myocardial infarction. Coron Artery Dis 2011;22:138-44. [PubMed]
- Azab B, Torbey E, Hatoum H, et al. Usefulness of red cell distribution width in predicting all-cause long-term mortality after non-ST-elevation myocardial infarction. Cardiology 2011;119:72-80. [PubMed]
- Ren H, Hua Q, Quan M, et al. Relationship between the red cell distribution width and the one-year outcomes in Chinese patients with stable angina pectoris. Intern Med 2013;52:1769-74. [PubMed]
- Yao HM, Sun TW, Zhang XJ, et al. Red blood cell distribution width and long-term outcome in patients undergoing percutaneous coronary intervention in the drug-eluting stenting era: a two-year cohort study. PLoS One 2014;9:e94887. [PubMed]
- Osadnik T, Strzelczyk J, Hawranek M, et al. Red cell distribution width is associated with long-term prognosis in patients with stable coronary artery disease. BMC Cardiovasc Disord 2013;13:113. [PubMed]
- Arbel Y, Birati EY, Finkelstein A, et al. Red blood cell distribution width and 3-year outcome in patients undergoing cardiac catheterization. J Thromb Thrombolysis 2014;37:469-74. [PubMed]
- Lappé JM, Horne BD, Shah SH, et al. Red cell distribution width, C-reactive protein, the complete blood count, and mortality in patients with coronary disease and a normal comparison population. Clin Chim Acta 2011;412:2094-9. [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [PubMed]