Sternochondral replacement: use of cadaveric allograft for the reconstruction of anterior chest wall
Introduction
The sternum can be involved or damaged by different diseases such as trauma, infection after cardiac surgery, tumors (primary and secondary) or chest wall deformities (1). When required, sternal resection and reconstruction represents the main treatment with the fundamental targets of a surgical excision with safety borders in case of tumors, prevention of respiratory impairment and defense of surrounding organs (2,3). In the last forty years many materials have been proposed for the sternal replacement, ranging from soft [e.g., polypropylene (PPM) or polytetrafluoroethylene (PTFE) meshes] (1,2,4-6), to rigid (e.g., plates of methacrylate, silicone, titanium and cyanoacrylate meshes) materials. Both these devices have advantages and drawbacks: soft mesh does not guarantee adequate chest wall stability and protection of surrounding organs and their softness provides low support to the respiratory dynamics, allowing paradoxical movements; stiff materials permit a better protection of the mediastinum, but they reduce chest wall flexibility, making it a fixed cage (7-9). In addition, infection risk is a serious problem particularly for soft and composite meshes requiring prosthesis removal (3,10). In recent years, the use of auto and allograft has been successfully adopted for sternal replacement: in particular, our group firstly illustrated the use of sternochondral allograft taken from cadaver to replace the sternum after an extensive resection for a primary chondrosarcoma (11). Herein, we describe our experience in sternal replacement with donor cryopreserved sternum allograft.
Methods
From February 2008 to October 2017, 14 patients were operated. Resection and reconstruction of sternum and anterior chest wall using cadaveric cryopreserved sternal allograft were done. Patients were 5 males and 9 females, with an average age of 61 years and a median age of 66.5 years (range, 35–78 years). The indications for this surgical procedure were: primary sternal neoplasm (in particular 6 cases of chondrosarcoma, 1 sarcoma interesting cervical region and mediastinum and 1 plasmocitoma), metastatic disease in a single location in 4 patients (breast carcinoma metastases in 3 cases and from liver carcinoma in 1 case), 1 sternal involvement from neuroendocrine thymic carcinoma and 1 sternal dehiscence after cardiac surgery.
The patient’s eligibility was defined by standard chest X-ray, a total body computed tomography (CT) or a chest CT-scan. In suspected cases of mediastinal or thoracic outlet involvement chest magnetic resonance (MRI) was performed. Every patient was routinely studied with cardiopulmonary tests. Eleven out of 13 patients affected by tumors performed pre-operative diagnosis, 9 of which by CT or ultrasound-guided needle biopsy and 2 by surgical incisional biopsy.
The average diameter of the tumor was 8 cm and the median major diameter was 6 cm (range, 3–21 cm). Three patients received induction therapy before operation (chemotherapy in 2 cases—soft tissue sarcoma and neuroendocrine thymic carcinoma—chemo-radiotherapy for the patient affected by breast-skin angiosarcoma).
Sternal graft
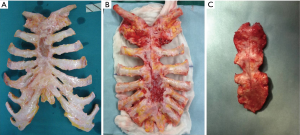
The sterno-chondral graft was reaped from a tissue bank under a complete aseptic technique according with the Italian legislation for the donation. A treatment with an antibiotic solution for 72 hours at +4 °C and the cryopreservation at −80 °C were performed in order to permit graft sterility and the absence of immunogenic capacity. The block was defrosted at 4–6 °C for 12 h since the day before surgery and, during the operation, it was taken out of sterile bags. Graft defrosting was completed in a 0.9% NaCl solution with antibiotics.
Surgical procedure (Figure 1)

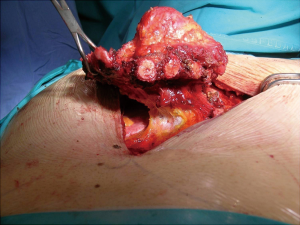
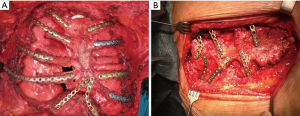
Patient position was supine and we performed a midline incision with removal of subcutaneous or cutaneous tissue with a 3 cm margin of macroscopically normal tissue. Usually, a muscle flap, generally of pectoralis major, was prepared and a partial or complete resection of the sternum en bloc with costal cartilages was performed (Figures 2,3). After the sternum removal and the complete defrosting of the allograft, surgeons proceeded with the cutting and tailoring of the block, in order to perfectly fit with the chest wall defect (Figure 4). Titanium plates were affixed to the sternal allograft to give stability (Synthes, Solothurn, Switzerland) (Figure 5). Depending on the situation, other devices were used: in partial lower sternal resections, the residual sternum was fixed with an H shaped plate to the allograft; in total or upper sternectomy, the new manubrium was fixed to clavicles with hi-tension polyethylene suture or with a titanium bar or with intramedullary nails (Synthes, Solothurn, Switzerland) (Figure 6). Muscle flaps are finally fixed medially to protect the graft.
Follow up
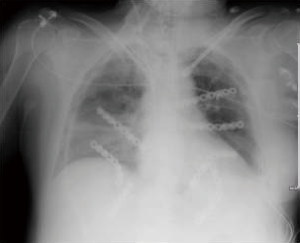
During follow up, patients underwent clinical visit, dosage of tumor markers and total body CT-scan with chest wall 3-D reconstruction every 6 months, with the aim to detect relapse of tumor or infection, and to check the stability and the correct position of the graft (Figure 7).
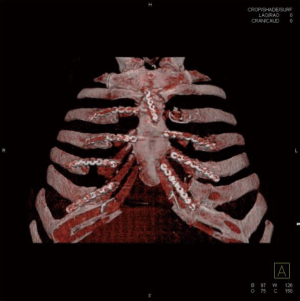
Results
Out of 14 procedures, 2 cases were total sternectomy, 7 were partial (lower) sternectomy, 5 cases were partial (upper) sternectomy involving the manubrium and part of the body (in one patient also clavicles were involved). One patient underwent lung wedge resection, and another superior vena cava system resection because of direct involvement of the primary disease. A muscle flap of pectoralis major was prepared to cover the graft in 9 patients (in 1 case both pectoralis muscles were used) and in 1 case a latissimus dorsi musculocutaneous flap was used. Adjuvant therapy was performed after surgery in three patients: in particular, 2 of them received chemotherapy and 1 of them radiotherapy.
Post-operative outcome
No postoperative complications happened in 11 cases (84.6%). One (7.1%) patient died 9 days after surgery because of pulmonary embolism. Two patients (15.4%) had complications: one presented fever caused by systemic candidiasis and one had a muscle flap bleeding. This patient received surgical revision of the flap. Complications regarding the allograft occurred in 2 cases: one patient underwent surgery to remove a displaced clavicular screw four months after surgery and the latter required a revision of the lower part of the surgical scar with removal of one titanium caudal plate for a wound dehiscence with exposure of bone graph and plates with no evidence of infection. In both cases the stability of the graft was perfectly preserved with no subsequent problems. The median hospitalization time was 11 days (range, 6–31 days). At an average follow up of 41 months (range, 6–137 months) on 13 patients, 7 patients were alive in absence of disease, 1 patient is alive with metatarsal plasmocitoma recurrence, 5 patients died (4 of systemic relapse of primary neoplasm, 1 of ictus) but nor infection neither rejection of the graft happened. No respiratory impairment or flail chest were registered in any patients. The patient with sternal dehiscence referred persistence of chronic pain even after sternal replacement.
Histology
Eleven (84.6%) out of 13 patients with neoplastic disease involving the sternum had R0 resection, one patient had R1 resection with microscopic residual disease evidenced at histological analysis and one patient with thymic carcinoma had a R2 resection with macroscopic residual mediastinal disease.
Discussion
Sternal resection following by chest wall reconstruction is still a challenging surgical procedure due to the absence of an ideal sternal substitute able to guarantee a safe protection of the mediastinal structure and the preservation of the respiratory function. A variety of different materials and techniques have been described for sternal replacement. The adoption of autogenous tissues date back to 1948, when Bisgard and Swenson (13) firstly described the use of an autogenous costal graft and, at the same time, Watson and James (14) recommended the use of fascia lata flap to cover chest wall defect; meanwhile Campbell et al. (15) reported the latissimus dorsi muscle flap use.
Considering the poor amount of available tissue and the donor site morbidity, prosthetic implantable materials [like prosthetic woven meshes, e.g., polytetrafluoroethylene (ePTFE), polypropylene (Marlex) or Prolene meshes] were proposed in the following years in order to repair the surface of the thoracic defect (16). Nevertheless, these materials demonstrated their inability to protect internal organs from an external impact. In the 1980s, rigid prostheses (polypropylene mesh or methylmethacrylate composite) were introduced: they increased chest wall stability and protection, but did not preserve the dynamic structure of the chest, transforming it into a fixe cage (7). Moreover, prosthetic materials might consume adjacent structures during respiratory movements, and rejection or infection might also occur (17). The recent introduction of other rigid devices such as titanium plates and screws were a further advancement with significant reduction of risk infection, but with the same problems of the other rigid materials and the additional risk of plate disruption or displacement (18-20).
Another significant progress was the use of cryopreserved bone allograft as alternative for chest wall reconstruction: Cara et al. (21) were pioneers in this technique in the 1993, publishing a case of reconstruction of the sternum using an allograft of the cryopreserved iliac bone and muscle-cutaneous flaps. Then, Rocco et al. (22) reported the use of cryopreserved iliac crest bone combined with different materials, creating an artificial chest cage after a huge demolition of the anterior chest wall for a recurrent chondrosarcoma.
The bone allografts represent a compromise in reconstructive surgery because of the advantages of the use of autogenous bones and prosthetic materials (e.g., rigidity, adaptability) without impending disadvantages (e.g., risk of infection or rejection). Moreover, compared with bone autografts, the harvesting of allografts is not cause of morbidity for the donor and, most notably, the amount of bone that can be obtained is not limited.
In 2010, we first illustrated the use of a sternochondral allograft taken from cadaver to repair a large chest wall defect after sternectomy (11). This technique showed significant advantages: the sternochondral allograft provides a rigid structure and a complete covering of the defect of the chest wall; furthermore, by cutting and tailoring the graft to perfectly replace the defect, it reproduces the original sternochondral plate, allowing satisfactory protection of the adjacent organs and a more physiological reconstruction of the chest cage. Another advantage of bone allograft is that no respiratory deficiency or complications related to insufficient or altered ventilation movements were recorded (23). Finally, last major advantage of bone grafts (auto- and allograft) compared to synthetic materials, is their capability of integration with the host patient’s living tissue, thanks to their osteoconductive and osteoinductive capacities (24). The graft acts as a scaffold for the genesis of new bone, permitting the ingrowth of capillaries and perivascular tissue into its structure and recruiting undifferentiated mesenchymal cells from adjacent tissue that can differentiate into osteoprogenitor cells under inductive stimulus of bone growth factors (25). These properties of the grafts also reduce the risk of infection and of immunologic reaction. The sterility of the graft and the absence of immunogenic capacity are allowed by cryopreservation.
In our study, at an average follow up of 41 months biocompatibility and mechanical characteristics of this material were established by the absence of complications related to the bone-allograft. Moreover, the use of titanium plates and screws to fix the graft has a number of benefits: firstly, they have an extraordinary tissue compatibility and can last in time; the titanium implants remain chemically inert and corrosion-free and can easily and precisely be adapted to the margins of the ribs; no allergic reactions caused by titanium have been found at this time; titanium reflects significantly less than steel, therefore it is more compatible with computer- and MRI-tomograms (26).
In our experience surgery was necessary only in one patient to remove a screw, because of partial displacement on the clavicle, 4 months after operation, without effects for the stability of the graft. In this case, the patient received a total sternectomy with sterno-clavicular joints reconstruction: probably the continuous clavicular articulation movements caused the dislocation of the screw.
In conclusion, the sternal allograft can be considered for sternal replacement as a safe and biologically well-tolerated solution. The use of this material has several benefits over synthetic materials and bone autografts with excellent results in terms of function, protection and aesthetic. We believe that our positive experience can favour a more widespread use of donor cryopreserved sternochondral allograft in the future as an ideal way for anterior chest wall reconstruction. Further studies would be useful to support this experience and to demonstrate the efficacy of this procedure.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study obtained ethics approval by the ethics committee of the University Hospital of Padova; the ID of the approval has not been obtained because of the retrospective nature of the study; all patients gave informed consent before taking part to the study, included informed consent to publish any accompanying intra-operative images and videos, in agreement with the rules of the local ethical committee.
References
- Arnold PG, Pairolero PC. Chest-wall reconstruction: an account of 500 consecutive patients. Plast Reconstr Surg 1996;98:804-10. [Crossref] [PubMed]
- Incarbone M, Pastorino U. Surgical treatment of chest wall tumors. World J Surg 2001;25:218-30. [Crossref] [PubMed]
- Mahabir RC, Butler CE. Stabilization of the chest wall: autologous and alloplastic reconstructions. Semin Plast Surg 2011;25:34-42. [Crossref] [PubMed]
- Mansour KA, Thourani VH, Losken A, et al. Chest wall resections and reconstruction: a 25-year experience. Ann Thorac Surg 2002;73:1720-5. [Crossref] [PubMed]
- McCormack PM. Use of prosthetic materials in chest-wall reconstruction. Assets and liabilities. Surg Clin North Am 1989;69:965-76. [Crossref] [PubMed]
- Deschamps C, Tirnaksiz BM, Darbandi R, et al. Early and long-term results of prosthetic chest wall reconstruction. J Thorac Cardiovasc Surg 1999;117:588-91. [Crossref] [PubMed]
- Girotti P, Leo F, Bravi F, et al. The "rib-like" technique for surgical treatment of sternal tumors: lessons learned from 101 consecutive cases. Ann Thorac Surg 2011;92:1208-15. [Crossref] [PubMed]
- Soysal O, Walsh GL, Nesbitt JC, et al. Resection of sternal tumors: extent, reconstruction, and survival. Ann Thorac Surg 1995;60:1353-8. [Crossref] [PubMed]
- Hasse J. Surgery for primary, invasive and metastatic malignancy of the chest wall. Eur J Cardiothorac Surg 1991;5:346-51. [Crossref] [PubMed]
- Chapelier AR, Missana MC, Couturaud B, et al. Sternal resection and reconstruction for primary malignant tumors. Ann Thorac Surg 2004;77:1001-6. [Crossref] [PubMed]
- Marulli G, Hamad AM, Cogliati E, et al. Allograft sternochondral replacement after resection of large sternal chondrosarcoma. J Thorac Cardiovasc Surg 2010;139:e69-70. [Crossref] [PubMed]
- Marulli G, De Iaco G, Ferrigno P, et al. Sternochondral resection for chondrosarcoma and reconstruction with cadaveric allograft. Asvide 2020;7:001. Available online: http://www.asvide.com/watch/33045
- Bisgard JD, Swenson SA Jr. Tumors of the sternum; report of a case with special operative technic. Arch Surg 1948;56:570-8. [Crossref] [PubMed]
- Watson WL, James AG. Fascia lata grafts for chest wall defects. J Thorac Surg 1947;16:399-406. [PubMed]
- Campbell DA. Reconstruction of the anterior thoracic wall. J Thorac Surg 1950;19:456-61. [PubMed]
- Watanabe A, Watanabe T, Obama T, et al. New material for reconstruction of the anterior chest wall, including the sternum. J Thorac Cardiovasc Surg 2003;126:1212-4. [Crossref] [PubMed]
- Aranda JL, Varela G, Benito P, Juan A. Donor cryopreserved rib allografts for chest wall reconstruction. Interact Cardiovasc Thorac Surg 2008;7:858-60. [Crossref] [PubMed]
- Weyant MJ, Bains MS, Venkatraman E, et al. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg 2006;81:279-85. [Crossref] [PubMed]
- Chai Y, Zhang G, Shen G. Autogenous rib grafts for reconstruction of sternal defects after partial resection: a new surgical technique. Plast Reconstr Surg 2008;121:353e-355e. [Crossref] [PubMed]
- Heller L, Huang WC, Chen HC, et al. Vascularized iliac bone flap used for sternum reconstruction after resection of chondrosarcoma. Plast Reconstr Surg 2002;110:1088-91. [PubMed]
- Cara JA, Laclériga AF, Cañadell J. Iliac allograft used for sternal reconstruction after resection of a chondrosarcoma. Int Orthop 1993;17:297-9. [Crossref] [PubMed]
- Rocco G, Fazioli F, Scognamiglio F, et al. The combination of multiple materials in the creation of an artificial anterior chest cage after extensive demolition for recurrent chondrosarcoma. J Thorac Cardiovasc Surg 2007;133:1112-4. [Crossref] [PubMed]
- Marulli G, Dell'amore A, Calabrese F, et al. Safety and Effectiveness of Cadaveric Allograft Sternochondral Replacement After Sternectomy: A New Tool for the Reconstruction of Anterior Chest Wall. Ann Thorac Surg 2017;103:898-905. [Crossref] [PubMed]
- Garcia-Tutor E, Yeste L, Murillo J, et al. Chest wall reconstruction using iliac bone allografts and muscle flaps. Ann Plast Surg 2004;52:54-60. [Crossref] [PubMed]
- Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osteointegration. Eur Spine J 2001;10:S96-101. [Crossref] [PubMed]
- De Palma A, Sollitto F, Loizzi D, et al. Chest wall stabilization and reconstruction: short and long-term results 5 years after the introduction of a new titanium plates system. J Thorac Dis 2016;8:490-8. [Crossref] [PubMed]