
Mechanisms of resistance to osimertinib
Introduction
The discovery of activating mutations in the tyrosine kinase (TK) domain of the epidermal growth factor receptor (EGFR) gene (1,2) has opened the era of stratified medicine in the treatment of patients with non-small cell lung cancer (NSCLC) (3).
Despite the high percentage of objective response rate (ORR) and the survival improvement observed in EGFR mutated patients receiving EGFR tyrosine kinase inhibitors (TKIs), complete responses are uncommon, and after a median of 8–18 months, acquired resistance mechanisms emerge, determining the development of patients’ progression (4). The use of comprehensive next generation sequencing (NGS) panels is helping to better define the genomic complexity of EGFR mutated NSCLC patients. Currently, EGFR mutated NSCLC is not considered as a homogenous tumor, driven by a common oncogenic event, but as a heterogeneous disease, including subsets of molecularly subcategorized patients. A higher somatic tumor mutation burden (TMB) was found in patients harboring EGFR L858R exon 21 mutation compared with those carrying exon 19 deletion. These data suggest the presence of heterogeneous preexisting subclones, that might emerge under pressure of TKI therapy, thus explaining the poor prognosis observed in patients harboring EGFR L858R mutation (5). In addition, concomitant molecular alterations that might influence patients’ prognosis and the sensitivity to treatment have been identified. Recently, the NGS MSK-IMPACT assay was used to classify 200 advanced EGFR mutated NSCLC patients with available pretreatment tissue and define the impact of co-occurring genetic alterations on prognosis (6). Concomitant mutations in TP53, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), catenin beta-1 (CTNNB1) and retinoblastoma (RB1) were observed. The presence of TP53 mutations was associated with shorter overall survival (OS) and progression free survival (PFS). Currently, the functional role of all TP53 mutations identified in EGFR mutated NSCLC has not been determined. However, loss of function mutations in TP53 and RB1, observed in approximately nine percent of EGFR mutated patients, increases the risk of small cell lung cancer (SCLC) transformation. Similarly, concomitant amplification in mesenchymal-epithelial transition factor (MET) or ERBB2 genes significantly reduces PFS in patients under EGFR-TKIs, thus suggesting the need to define customized combinatorial treatment strategy (6). The recent approval of osimertinib, an orally available, irreversible inhibitor of EGFR-sensitizing and T790M-resistant mutations, has improved the therapeutic armamentarium in EGFR mutated NSCLC patients (7). Based on its favorable toxicity profile, osimertinib is considered a particularly attractive candidate for combinatorial approaches that may potentially improve the clinical outcome of this subset of patients.
The current review focuses on the clinical data leading to the approval of osimertinib and provides an overview of the mechanisms of acquired resistance to osimertinib and the ongoing therapeutic strategies to overcome resistance.
Clinical development of osimertinib
Osimertinib was originally designed to target EGFR sensitizing mutations and T790M resistant mutation, that represents the most common acquired resistance mechanism to first and second-generation EGFR-TKIs (8). Osimertinib irreversibly binds to the cysteine-797 located in the adenosine triphosphate (ATP) binding site within the TK domain of the EGFR, while sparing the wild-type form of the receptor (8,9). It inhibits proliferation of cell lines harboring EGFR sensitizing and T790M mutations, and determines significant tumor regression in mutant xenograft models and transgenic tumor models carrying these alterations.
Moreover, osimertinib was designed to penetrate the blood brain barrier (BBB), since gefitinib, erlotinib and afatinib have unfavorable physicochemical properties to achieve an effective exposure in the central nervous system (CNS), due to the presence of two hydrogen bond donors in the case of afatinib and multiple rotatable bonds (10 for gefitinib and 11 for erlotinib), that limit the CNS penetration (10). Despite the fact that osimertinib is a substrate of P-glycoprotein and breast cancer resistance protein (BCRP) efflux transporters, which remove drugs from the CNS, its permeability is sufficient to overcome the efflux, thus suggesting its potential efficacy on brain metastases (11). Preclinical data indicate that osimertinib distribution at mouse brain is greater compared to gefitinib, erlotinib or afatinib, and determines brain tumor regression in EGFR mouse brain metastases model (11).
Based on these preclinical data, osimertinib entered in clinical development. The phase I AURA trial (Table 1) evaluated the pharmacokinetic profile and efficacy of osimertinib in EGFR mutated patients with radiological documented progression to previous EGFR-TKIs (12). Osimertinib was well tolerated and no dose limiting toxicity was registered. The dose of 80 mg once daily was selected for the further clinical development. Partial responses were observed in 51% of the enrolled patients, with a disease control rate of 84%. ORR increased to 61% when only T790M positive patients were considered. These results were confirmed in the phase II AURA2 study (Table 1), that enrolled patients progressing to prior EGFR-TKI therapy, with centrally confirmed T790M resistance mutation (13).
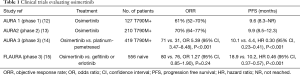
Full table
An ORR of 70% was registered, with a median PFS of approximately 10 months, leading to the accelerated approval of osimertinib in 2015 by the food and drug administration (FDA) for the treatment of advanced EGFR T790M mutation positive NSCLC progressing to EGFR-TKIs. Recently, a long-term follow-up of a pooled analysis including results from the AURA and the AURA2 trials, showed a median OS of 26.8 months, with OS rates at 12, 24, and 36 months of 80%, 55% and 37%, respectively (16). Full approval of osimertinib by FDA was granted in March 2017 based on the results from the Phase III AURA3 study (Table 1), demonstrating in 419 T790M-positive advanced NSCLC patients, who had progressed to first-line EGFR-TKIs, the superiority of osimertinib over platinum-pemetrexed chemotherapy in terms of PFS and ORR, thus establishing osimertinib as the standard of care in this setting (14). Osimertinib significantly prolonged PFS also in the subgroup of patients with brain metastases. One hundred sixteen out of 419 patients had measurable and/or non-measurable CNS lesions (17). CNS ORR was significantly higher in patients receiving osimertinib, compared to those under chemotherapy (70% vs. 31%, respectively). Moreover, radiological responses were registered in cases with leptomeningeal carcinomatosis, therefore demonstrating the effective CNS penetration of the drug. The CNS activity of osimertinib has been confirmed in a pooled analysis, including data from 194 patients enrolled in the two phase II studies, the AURA extension and the AURA2 (18). Of these, 74% had previously received brain radiotherapy. A CNS ORR of 54%, with a CNS disease-control rate (DCR) of 92% was observed. Conversely from AURA3 results, where the CNS efficacy of osimertinib was higher in those patients receiving brain radiation in the previous 6 months, radiotherapy did not affect brain disease control in the patients included in the pooled analysis. Thanks to the good efficacy and the tolerability profile observed in pre-treated patients, the phase III FLAURA study (Table 1) was designed to explore PFS improvement of osimertinib over first-generation EGFR-TKIs in EGFR mutated, treatment-naive NSCLC patients (15). One hundred fifty six NSCLC patients, stratified according to the type of EGFR mutation (exon 19 deletion or L858R) and race, were randomized to receive osimertinib or gefitinib/ erlotinib. Cross-over to osimertinib was allowed at disease progression in patients receiving standard EGFR-TKI after documentation of T790M-positive mutation status on plasma or tissue. Results demonstrated prolonged PFS with osimertinib over standard EGFR-TKI, with a benefit observed across all predefined subgroups of patients, including those with CNS metastases. A significantly lower percentage of CNS progression was observed in the osimertinib arm compared with first generation-TKI (20% vs. 39%) (19). A lower rate of grade ≥3 adverse events (AEs) and of AEs leading to treatment discontinuation was reported in patients receiving osimertinib, despite the longer time of exposure to this drug, suggesting a better tolerability profile. Based on these results, in April 2018, osimertinib indication was extended as first-line treatment of EGFR mutated NSCLC patients, thus improving the therapeutic opportunities and opening new questions regarding its use up-front or at time of disease progression following treatment with first- or second-generation EGFR-TKIs.
Acquired mechanisms of resistance and strategies to overcome resistance
Multiple biological mechanisms of acquired resistance to osimertinib have been identified, thus confirming tumor heterogeneity and adaptability (Table 2). These mechanisms include secondary resistance mutations, that interfere with drug binding, the most frequent being EGFR C797S, or the activation of alternative signaling pathways (20,21). The analysis of cell free DNA (cfDNA) by NGS, performed in plasma from a subgroup of patients enrolled in the phase I AURA trial, who had progressed to osimertinib, revealed the presence of C797S mutation in the exon 20 of the EGFR gene in approximately 40% of the patients evaluated (22). Osimertinib forms a covalent bond with the cysteine 797 located at the ATP binding pocket, and mutation at this site prevents the binding of the drug, thus conferring resistance. Preclinical findings demonstrate that the efficacy of first and second-generation TKIs is not affected by the cysteine at position 797 (23,24), thus suggesting that treatment with these drugs might be a strategy to overcome EGFR C797S resistance mutation acquired following osimertinib. However, due to the concurrent T790M mutation in patients previously treated with first or second-generation inhibitors, a combinatorial treatment with osimertinib and a first or second generation-TKI is required to overcome resistance. In this context, the configuration of the T790M and C797S mutations affects how tumor cells could respond to therapy. When the two mutations are on different alleles (in trans), the combination of first- and third-generation TKIs can restore EGFR inhibition (24). Conversely, the presence of the two mutations on the same allele (in cis) confers resistance to all generations of EGFR-TKIs, therefore suggesting the need for alternative treatment strategies (24). Recently, tumor re-biopsy was performed in 41 EGFR T790M-positive patients at the time of osimertinib progression (25). Loss of T790M was observed in 68% of cases. Among these, small-cell lung cancer transformation was identified in approximately 21% of patients. In the other cases, NGS revealed MET amplifications, PIK3CA, Kirsten Rat Sarcoma Viral oncogene homolog (KRAS) or BRAF mutations, rearranged during transfection (RET), fibroblast growth factor receptor 3 (FGFR3), and BRAF fusions. EGFR C797S was observed in 22% of patients, 69% of whom maintained the T790M, all in cis. In some cases, the analysis of plasma by NGS was discordant with the molecular results identified in tissue, since plasma revealed additional alterations, such as anaplastic lymphoma kinase (ALK) rearrangement in one patient and EGFR G724S in another. Time to treatment discontinuation to osimertinib was longer in patients who maintained the T790M. Conversely, shorter responses were observed in the case of T790M loss, therefore indicating a more heterogeneous tumor, in which T790M clones co-exist with other resistance mechanisms, that might confer early resistance and a more aggressive phenotype (25). Similar findings were observed in 41 osimertinib-resistant patients treated at Massachusetts General Hospital (26). The EGFR C797S in cis configuration with T790M was found in 19% of cases, 22% developed MET amplification, while 38% lost the T790M mutation. Additionally, the CCDC6–RET fusion was identified. The selective RET inhibitor BLU-667, that showed to be 15 times more potent to inhibit RET than any other kinase, and >10 times more potent on RET compared to other approved RET inhibitors, was combined with osimertinib in a patient who developed CCDC6–RET fusion following osimertinib progression. A remarkable tumor reduction was observed with a good tolerability profile (26).
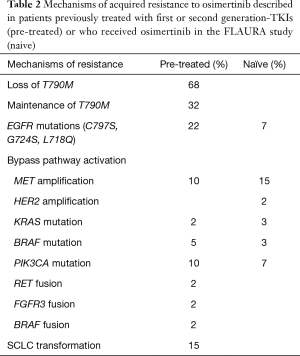
Full table
Recently, the EGFR G742S mutation, located at exon 18, has been described in patients developing tumor progression in the course of osimertinib (27,28). It is less frequent compared to EGFR C797S, and mutually exclusive with EGFR C797S. The available data indicate that the G742S might emerge or persist in osimertinib-resistant clones that evolve independently of acquired EGFR T790M mutations. Preclinical findings demonstrated the lack of efficacy of osimertinib in double-mutant EGFR19del+G724S xenograft mouse models (28). The G724S is located in the glycine-rich loop in the N-lobe of the kinase domain, which is critical for substrate and ligand binding. EGFR G724S alters fluctuations of the glyicine-rich loop, thus preventing the reversible binding of osimertinib within the ATP pocket, required before the attachment to cysteine 797. Conversely, afatinib has been shown to reversible bind the ATP-pocket despite the EGFR G724S mutation, therefore representing a therapeutic strategy to overcome resistance. The combination of osimertinib and afatinib determined a remarkable response in a patient with acquired EGFR G724S mutation (29). An additional acquired mechanism described is represented by the development of the ALK rearrangement. Brigatinib combined with osimertinib determined a significant radiological and symptomatic improvement. Beyond secondary mutations, activation of bypass tracks can also be responsible for resistance to osimertinib. Preclinical reports using osimertinib have demonstrated bypass activation of the mitogen-activated protein kinase (MAPK) ERK-RAS pathway through different mechanisms, including MAPK1 amplification, down-regulation of negative regulators of ERK, NRAS mutation/amplification, KRAS amplification among others, thus suggesting that a combinatorial treatment using osimertinib with a MEK inhibitor might potentially prevent acquired resistance in some cases (30).
Following the results of the FLAURA study and the introduction of osimertinib in the first-line setting, the treatment strategy at osimertinib progression remains an open question. To determine the molecular mechanisms of acquired resistance in patients receiving osimertinib in the front-line setting, and to define whether alternative mechanisms occur compared to those observed in patients receiving osimertinib following first or second generation-TKI, plasma from patients enrolled in the FLAURA trial, who developed progression under osimertinib, was analyzed by NGS. No evidence of acquired T790M was observed. The most common resistance mechanisms resulted to be the C797S mutation (7%) and MET amplification (15%). Other mechanisms included HER2 amplification, PIK3CA, KRAS, BRAF or rare EGFR secondary mutations. Based on these findings, different phase I/II trials are exploring the safety of combinatorial approaches, including osimertinib and targeted agents directed against the activated bypass signaling pathway.
The TATTON (NCT02143466) was a phase Ib study, exploring different osimertinib combinations according to the acquired resistance mechanisms. It evaluated osimertinib and the MET inhibitor, savolitinib, in patients with MET-positive tumor, osimertinib and the MEK inhibitor, selumetinib, in those carrying KRAS mutations, osimertinib and the programmed death ligand 1 (PD-L1) inhibitor, durvalumab, in those patients with no molecular alteration identified. The combination of savolitinib and osimertinib was explored in two cohorts of patients, including those with EGFR-mutant lung cancer, T790M negative, with acquired resistance driven by MET amplification following treatment with a first- or second-generation EGFR TKI, and those with EGFR-mutant lung cancer with acquired resistance driven by MET amplification after treatment with osimertinib or another experimental third-generation EGFR TKI. In the cohort of 46 patients who had received prior first- or second-generation EGFR TKI, treatment with osimertinib plus savolitinib yielded an ORR of 52%, while among the 48 MET-positive patients with disease progression following osimertinib, the ORR was 28%, with a median duration of response of 9.7 months (31). Three different schedules of osimertinib and selumetinib were evaluated in KRAS mutated patients: two included continuous administration of osimertinib, associated with selumetinib at the dose of 25 mg twice daily or 50 mg twice daily. The third regimen evaluated osimertinib combined with selumetinib at the dose of 75 mg twice daily on the first and fourth day of each week. The ORR was 17% for those patients who had received prior treatment with osimertinib. In the dose-expansion phase, the escalated intermittent regimen was used. An ORR of 23% was registered. Finally, the combination of durvalumab and osimertinib was prematurely stopped due to the high incidence of interstitial lung disease (ILD)-like events, observed in 38% of enrolled patients (32).
Albeit the impressive efficacy of osimertinib in naïve EGFR-mutated NSCLC patients, the activation of compensatory signaling pathways, including SRC family kinases (SFKs), focal adhesion kinase (FAK) and the up-regulation of receptor tyrosine kinases (RTKs) might occur, thus contributing to osimertinib resistance (33). Phosphorylation of signal transducer and activator of transcription (STAT3) and Src-YES-associated protein 1 (YAP1) has been observed following osimertinib (34). Once activated, STAT3 and YAP1 translocate into the nucleus and promote gene transcription. Recently, over-expression of AXL (35), which is a downstream target of YAP1, and CUB domain-containing protein-1 (CDCP1) has been observed in EGFR TKI-naive patients (33). The combination of osimertinib with the Src/FAK/Janus kinase 2 (JAK2) inhibitor, TPX-0005, abrogated STAT3, YAP1 and SFKs activation and down-regulated AXL and CDCP1 expression in vitro and in vivo, therefore suggesting the potential efficacy of this combination to induce more durable responses in patients receiving osimertinib.
Commentary
The recent approval of osimertinib as fist-line treatment of patients with EGFR mutated NSCLC has opened new questions. Phase III studies have demonstrated that gefitinib, erlotinib, afatinib and osimertinib have a good tolerability profile, and determine significant PFS improvement. First and second generation-TKIs have significantly prolonged PFS over platinum doublet, without any impact on OS due to the cross-over effect. The pooled analysis from patients enrolled in the Lux-Lung 3 and Lux-Lung 6 trials indicated that afatinib significantly prolonged OS over chemotherapy in patients carrying exon 19 deletion (36). The PFS improvement of osimertinib over first generation-TKIs observed in the FLAURA study could be related to its efficient penetration of the BBB, that significantly improves disease control at brain site and prevents brain metastases development. However, the early separation of the Kaplan-Meyer curves, documented at the first computed tomography scan evaluation, might also be dependent on the efficient inhibition of osimertinib on pretreatment EGFR T790M. It is documented that a subpopulation of patients carry EGFR T790M at baseline. Different frequencies have been reported, depending on the detection methods used. Using a highly sensitive method based on peptide nucleic acid clamping PCR, T790M was observed in approximately 65% of baseline tumor samples from patients enrolled in the EURTAC study (37), and was associated with shorter PFS. Pretreatment T790M was not evaluated in patients enrolled in the FLAURA study. The ongoing phase II AZENT trial (NCT02841579) explores osimertinib in the subgroup of EGFR mutated naive NSCLC patients carrying concurrent pretreatment T790M mutations. Available data from liquid biopsy performed in patients from FLAURA developing progression under osimertinib, indicate that up-front osimertinib prevents T790M-mediated resistance, as no evidence of T790M was identified, thus confirming the efficient inhibition of T790M clones. Another open question remains the effect of osimertinib in patients carrying EGFR uncommon mutations. Currently there are only few reports, and it is not possible to draw a conclusion. Waiting for definitive OS data from FLAURA, it is currently unknown if osimertinib up-front is superior or comparable to a sequential strategy (first or second generation-TKI followed by osimertinib). The phase II APPLE trial (EORTC 1613) has been designed to address this issue. A total of 156 EGFR mutated NSCLC patients will be randomized between osimertinib, or gefitinib followed by osimertinib at the time of the emergence of T790M in blood independent of RECIST progression, or gefitinib followed by osimertinib at the time of disease progression. Data from literature indicate that EGFR T790M mutations can be identified in liquid biopsy at a median time of approximately 2 months before RECIST progression. Currently, the biological and clinical value of this finding is still unknown.
Conclusions
Although great improvements for the treatment of EGFR addicted NSCLC have been made in the last few years, the final goal of turning this disease into a chronic disease is still far from being reached. Targeted therapy is effective, but heterogeneity, clonal evolution, and selective pressures favor the development of acquired resistance mechanisms, determining tumor progression. Chemotherapy still plays an important role in the treatment of EGFR mutated NSCLC, especially in those cases developing mechanisms of acquired resistance not targetable by available compounds. Combinatorial treatment strategies are warranted to improve patients’ outcome.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Trever G. Bivona) for the series “Mechanisms of Resistance to EGFR-targeted Therapy” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2019.08.30). The series “Mechanisms of Resistance to EGFR-targeted Therapy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [Crossref] [PubMed]
- Rosell R, Karachaliou N. Large-scale screening for somatic mutations in lung cancer. Lancet 2016;387:1354-6. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Offin M, Rizvi H, Tenet M, et al. Tumor Mutation Burden and Efficacy of EGFR-Tyrosine Kinase Inhibitors in Patients with EGFR-Mutant Lung Cancers. Clin Cancer Res 2019;25:1063-9. [Crossref] [PubMed]
- Yu HA, Suzawa K, Jordan E, et al. Concurrent Alterations in EGFR-Mutant Lung Cancers Associated with Resistance to EGFR Kinase Inhibitors and Characterization of MTOR as a Mediator of Resistance. Clin Cancer Res 2018;24:3108-18. [Crossref] [PubMed]
- Gregorc V, Lazzari C, Karachaliou N, et al. Osimertinib in untreated epidermal growth factor receptor (EGFR)-mutated advanced non-small cell lung cancer. Transl Lung Cancer Res 2018;7:S165-70. [Crossref] [PubMed]
- Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046-61. [Crossref] [PubMed]
- Finlay MR, Anderton M, Ashton S, et al. Discovery of a potent and selective EGFR inhibitor (AZD9291) of both sensitizing and T790M resistance mutations that spares the wild type form of the receptor. J Med Chem 2014;57:8249-67. [Crossref] [PubMed]
- Zeng Q, Wang J, Cheng Z, et al. Discovery and Evaluation of Clinical Candidate AZD3759, a Potent, Oral Active, Central Nervous System-Penetrant, Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor. J Med Chem 2015;58:8200-15. [Crossref] [PubMed]
- Ballard P, Yates JW, Yang Z, et al. Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-Mutant NSCLC Brain Metastases Models, and Early Evidence of Clinical Brain Metastases Activity. Clin Cancer Res 2016;22:5130-40. [Crossref] [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2016;17:1643-52. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Ahn MJ, Tsai CM, Shepherd FA, et al. Osimertinib in patients with T790M mutation-positive, advanced non-small cell lung cancer: Long-term follow-up from a pooled analysis of 2 phase 2 studies. Cancer 2019;125:892-901. [Crossref] [PubMed]
- Wu YL, Ahn MJ, Garassino MC, et al. CNS Efficacy of Osimertinib in Patients With T790M-Positive Advanced Non-Small-Cell Lung Cancer: Data From a Randomized Phase III Trial (AURA3). J Clin Oncol 2018;36:2702-9. [Crossref] [PubMed]
- Goss G, Tsai CM, Shepherd FA, et al. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: pooled data from two Phase II trials. Ann Oncol 2018;29:687-93. [Crossref] [PubMed]
- Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Ortiz-Cuaran S, Scheffler M, Plenker D, et al. Heterogeneous Mechanisms of Primary and Acquired Resistance to Third-Generation EGFR Inhibitors. Clin Cancer Res 2016;22:4837-47. [Crossref] [PubMed]
- Le X, Puri S, Negrao MV, et al. Landscape of EGFR-Dependent and -Independent Resistance Mechanisms to Osimertinib and Continuation Therapy Beyond Progression in EGFR-Mutant NSCLC. Clin Cancer Res 2018;24:6195-203. [Crossref] [PubMed]
- Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015;21:560-2. [Crossref] [PubMed]
- Godin-Heymann N, Ulkus L, Brannigan BW, et al. The T790M "gatekeeper" mutation in EGFR mediates resistance to low concentrations of an irreversible EGFR inhibitor. Mol Cancer Ther 2008;7:874-9. [Crossref] [PubMed]
- Niederst MJ, Hu H, Mulvey HE, et al. The Allelic Context of the C797S Mutation Acquired upon Treatment with Third-Generation EGFR Inhibitors Impacts Sensitivity to Subsequent Treatment Strategies. Clin Cancer Res 2015;21:3924-33. [Crossref] [PubMed]
- Oxnard GR, Hu Y, Mileham KF, et al. Assessment of Resistance Mechanisms and Clinical Implications in Patients With EGFR T790M-Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol 2018;4:1527-34. [Crossref] [PubMed]
- Piotrowska Z, Isozaki H, Lennerz JK, et al. Landscape of Acquired Resistance to Osimertinib in EGFR-Mutant NSCLC and Clinical Validation of Combined EGFR and RET Inhibition with Osimertinib and BLU-667 for Acquired RET Fusion. Cancer Discov 2018;8:1529-39. [Crossref] [PubMed]
- Oztan A, Fischer S, Schrock AB, et al. Emergence of EGFR G724S mutation in EGFR-mutant lung adenocarcinoma post progression on osimertinib. Lung Cancer 2017;111:84-7. [Crossref] [PubMed]
- Fassunke J, Muller F, Keul M, et al. Overcoming EGFR(G724S)-mediated osimertinib resistance through unique binding characteristics of second-generation EGFR inhibitors. Nat Commun 2018;9:4655. [Crossref] [PubMed]
- Peled N, Roisman LC, Miron B, et al. Subclonal Therapy by Two EGFR TKIs Guided by Sequential Plasma Cell-free DNA in EGFR-Mutated Lung Cancer. J Thorac Oncol 2017;12:e81-4. [Crossref] [PubMed]
- Eberlein CA, Stetson D, Markovets AA, et al. Acquired Resistance to the Mutant-Selective EGFR Inhibitor AZD9291 Is Associated with Increased Dependence on RAS Signaling in Preclinical Models. Cancer Res 2015;75:2489-500. [Crossref] [PubMed]
- Sequist LV, Lee J, Han JY, et al. TATTON Phase Ib expansion cohort: osimertinib plus savolitinib for patients (pts) with EGFR-mutant, MET-amplified NSCLC after progression on prior third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI). Cancer Res 2019;79:Abstract nr CT033.
- Ahn MJ, Yang J, Yu H, et al. 136O: Osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: Results from the TATTON phase Ib trial. J Thorac Oncol 2016;11:S115. [Crossref]
- Karachaliou N, Chaib I, Cardona AF, et al. Common Co-activation of AXL and CDCP1 in EGFR-mutation-positive Non-smallcell Lung Cancer Associated With Poor Prognosis. EBioMedicine 2018;29:112-27. [Crossref] [PubMed]
- Chaib I, Karachaliou N, Pilotto S, et al. Co-activation of STAT3 and YES-Associated Protein 1 (YAP1) Pathway in EGFR-Mutant NSCLC. J Natl Cancer Inst 2017;109. [Crossref] [PubMed]
- Taniguchi H, Yamada T, Wang R, et al. AXL confers intrinsic resistance to osimertinib and advances the emergence of tolerant cells. Nat Commun 2019;10:259. [Crossref] [PubMed]
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. [Crossref] [PubMed]
- Costa C, Molina MA, Drozdowskyj A, et al. The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the randomized phase III EURTAC trial. Clin Cancer Res 2014;20:2001-10. [Crossref] [PubMed]


