
Preoperative variables for predicting prolonged air leak following pulmonary resection
We read with great interest the study by Murakami et al. recently published in Ann Thorac Surg. regarding grading of emphysema for predicting a prolonged air leak (PAL) following a lobectomy (1). One of the most common causes of complications and protracted hospital stay in patients who have received a pulmonary resection is PAL, which can cause distress, anxiety, and pain, as well as other complications, and also increases the risk of other cardiopulmonary complications and empyema. Therefore, management of pulmonary air leakage is an important clinical issue for thoracic surgeons (2,3). A variety of studies have reported the incidence of PAL and several have developed tools to calculate risk in order to identify patients at high risk using preoperative variables (Table 1). Generally, patients with PAL are more likely to be older, male, and smokers, as well as have peripheral vascular disease and a history of steroid use, along with a more fragile lung parenchyma with reduced healing capacity and lower body mass index (BMI), a marker of poor nutrition status. Furthermore, several authors who focused on preoperative pulmonary function testing have revealed that the ratio of lower forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) and diffuse capacity of carbon monoxide (DLCO) are independent risk factors for predicting PAL (3,13,14). Those results revealed that the most consistent risk factor is chronic obstructive pulmonary disease (COPD), as reflected by most of the risk factors noted above. Some have also created an aggregate risk score to stratify the incidence of PAL in patients who have undergone a pulmonary resection (3,4,8,9). Reliable information regarding the risk of PAL is very helpful to inform attending physicians regarding useful intraoperative preventative measures to minimize PAL occurrence, such as pleural testing and use of surgical sealants (9).
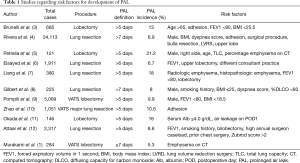
Full table
Murakami and colleagues focused on quantitative computed tomography (CT)-based grading of emphysema for predicting PAL after a lobectomy. Presently, whole-lung CT is routinely performed prior to lung cancer surgery. An “emphysema index” value can be calculated based on the volume of the total lung and that of the emphysematous lung, two parameters nearly automatically obtained using computer-based evaluation of whole-lung CT scans (1). Thus, calculation of that index seems to be easy to perform and the results obtained reasonable for predicting PAL before surgery. In univariate analysis findings, they also found that the “emphysema index” value was a significant predictor of the length of drainage and that remained significant in multivariate analysis results, thus was concluded to be a reliable predictor of PAL. Although previous reports noted that preoperative CT quantification of emphysema is one of the best predictors of PAL (5,7), the study by Murakami et al. describes how to define “emphysema index” as the proportion of emphysematous lung volume (<−910 HU) to total lung volume (−600 to −1,024 HU), thus showing the index to be reproducible and allowing for widespread adoption by thoracic surgeons. In addition to emphysema grade, physiological considerations may suggest that incomplete fissures and inflammation changes around pulmonary vessels and the bronchus are additional synergistic risk factors for PAL. Therefore, it is important to consider evaluation of CT findings, including emphysema severity, distribution, and fissure integrity, in order to provide important information for identification of patients at high risk for PAL in the future.
Surgical procedures such as an upper lobectomy and bilobectomy have been reported to increase the risk for developing PAL, because a larger residual pleural space precludes parietal-visceral pleural apposition (6). Other surgical factors reported to be correlated with PAL include a lobectomy rather than a wedge resection or segmentectomy (15), right-sided rather than left-sided resection (5), upper rather than lower or middle lobectomy (4), thoracotomy rather than video-assisted thoracoscopic surgery (16), and a primary surgeon with a high annual caseload (12). In addition, several studies have noted that detection of the presence of severe pleural adhesions is a relevant risk factor for PAL because of lung parenchyma injuries that occur during division of those adhesions (3,4), while factors related to the surgical technique employed are also important to prevent PAL during a pulmonary resection. The fissureless technique, in which dissection of the lung parenchyma over the pulmonary artery is avoided, appears to be a superior approach for patients with incomplete fissures (17), and use of surgical sealants and buttressing with or without fibrin sealant for air leakage after a pulmonary resection has been examined (18,19). A recent study found that placement of a free subcutaneous fat pad during a pulmonary resection reduced the duration of air leakage and chest tube drainage (20). Although these findings suggest that occurrence of air leaks is the result of multiple factors related to patient and disease characteristics, as well as the specific surgical technique used, evaluation of preoperative risk factors for PAL provides a more meaningful method of risk assessment prior to surgery, thus allowing selection of patients with a high risk for PAL who may benefit most from intraoperative preventative measures to reduce its occurrence. Nevertheless, additional investigations are needed to determine more effective intraoperative preventative measures in patients with an elevated risk for PAL.
In conclusion, CT-based grading of emphysema described by Murakami et al. (1) may be useful for identification of patients at high risk for PAL. Thoracic surgeons should validate those predictive models prospectively, and make an effort to reduce occurrence of PAL for high risk patients via intraoperative preventative measures.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Murakami J, Ueda K, Tanaka T, et al. Grading of Emphysema Is Indispensable for Predicting Prolonged Air Leak After Lung Lobectomy. Ann Thorac Surg 2018;105:1031-7. [Crossref] [PubMed]
- Burt BM, Shrager JB. Prevention and management of postoperative air leaks. Ann Cardiothorac Surg 2014;3:216-8. [PubMed]
- Brunelli A, Cassivi SD, Halgren L. Risk factors for prolonged air leak after pulmonary resection. Thorac Surg Clin 2010;20:359-64. [Crossref] [PubMed]
- Rivera C, Bernard A, Falcoz PE, et al. Characterization and prediction of prolonged air leak after pulmonary resection: a nationwide study setting up the index of prolonged air leak. Ann Thorac Surg 2011;92:1062-8; discussion 1068. [Crossref] [PubMed]
- Petrella F, Rizzo S, Radice D, et al. Predicting prolonged air leak after standard pulmonary lobectomy: computed tomography assessment and risk factors stratification. Surgeon 2011;9:72-7. [Crossref] [PubMed]
- Elsayed H, McShane J, Shackcloth M. Air leaks following pulmonary resection for lung cancer: is it a patient or surgeon related problem? Ann R Coll Surg Engl 2012;94:422-7. [Crossref] [PubMed]
- Liang S, Ivanovic J, Gilbert S, et al. Quantifying the incidence and impact of postoperative prolonged alveolar air leak after pulmonary resection. J Thorac Cardiovasc Surg 2013;145:948-54. [Crossref] [PubMed]
- Gilbert S, Maghera S, Seely AJ, et al. Identifying Patients at Higher Risk of Prolonged Air Leak After Lung Resection. Ann Thorac Surg 2016;102:1674-9. [Crossref] [PubMed]
- Pompili C, Falcoz PE, Salati M, et al. A risk score to predict the incidence of prolonged air leak after video-assisted thoracoscopic lobectomy: An analysis from the European Society of Thoracic Surgeons database. J Thorac Cardiovasc Surg 2017;153:957-65. [Crossref] [PubMed]
- Zhao K, Mei J, Xia C, et al. Prolonged air leak after video-assisted thoracic surgery lung cancer resection: risk factors and its effect on postoperative clinical recovery. J Thorac Dis 2017;9:1219-25. [Crossref] [PubMed]
- Okada S, Shimada J, Kato D, et al. Prolonged air leak following lobectomy can be predicted in lung cancer patients. Surg Today 2017;47:973-9. [Crossref] [PubMed]
- Attaar A, Winger DG, Luketich JD, et al. A clinical prediction model for prolonged air leak after pulmonary resection. J Thorac Cardiovasc Surg 2017;153:690-9.e2. [Crossref] [PubMed]
- Cerfolio RJ, Bass CS, Pask AH, et al. Predictors and treatment of persistent air leaks. Ann Thorac Surg 2002;73:1727-30; discussion 1730-1.
- Singhal S, Ferraris VA, Bridges CR, et al. Management of alveolar air leaks after pulmonary resection. Ann Thorac Surg 2010;89:1327-35. [Crossref] [PubMed]
- Stolz AJ, Schutzner J, Lischke R, et al. Predictors of prolonged air leak following pulmonary lobectomy. Eur J Cardiothorac Surg 2005;27:334-6. [Crossref] [PubMed]
- Yoo A, Ghosh SK, Danker W, et al. Burden of air leak complications in thoracic surgery estimated using a national hospital billing database. Clinicoecon Outcomes Res 2017;9:373-83. [Crossref] [PubMed]
- Gómez-Caro A, Calvo MJ, Lanzas JT, et al. The approach of fused fissures with fissureless technique decreases the incidence of persistent air leak after lobectomy. Eur J Cardiothorac Surg 2007;31:203-8. [Crossref] [PubMed]
- Malapert G, Hanna HA, Pages PB, et al. Surgical sealant for the prevention of prolonged air leak after lung resection: meta-analysis. Ann Thorac Surg 2010;90:1779-85. [Crossref] [PubMed]
- Ueda K, Tanaka T, Jinbo M, et al. Sutureless pneumostasis using polyglycolic acid mesh as artificial pleura during video-assisted major pulmonary resection. Ann Thorac Surg 2007;84:1858-61. [Crossref] [PubMed]
- Shintani Y, Inoue M, Funaki S, et al. Clinical usefulness of free subcutaneous fat pad for reduction of intraoperative air leakage during thoracoscopic pulmonary resection in lung cancer cases. Surg Endosc 2015;29:2910-3. [Crossref] [PubMed]


