
Treatment of anaemia in the “ERAS” era: how far can we go?
It is well-established that pre-operative anaemia, major bleedings and red blood cell (RBC) transfusions are risk factors for perioperative mortality, especially after cardiac surgery (1). It has been estimated an important economic saving (about US $78–97 million) when preoperative measures are undertaken for treating anaemia compared to the use of RBC transfusion during hospital stay (2,3). Management of iron deficiency and anaemia in the perioperative setting is still a matter of debate and several authors have tried to define different strategies.
The World Health Organization has classified postoperative anaemia as mild [when level of haemoglobin (hb) range from 11 to 12.9 g/dL], moderate (from 8 to 10.9 g/dL) or severe (lower than 8 g/dL). In 2018, Muñoz et al. (2) reported the results of the international consensus statement on the management of post-operative anaemia following major surgical procedures and advocated further researches on this issue, especially in the setting of management strategies or cost-effectiveness analysis of post-operative anaemia correction.
To answer these questions, Spahn et al. (4) performed a single center, randomised, double blind, parallel-group controlled study in which 484 patients undergoing cardiac surgery with anaemia (hb <12 g/dL) or isolated iron deficiency (ferritin level <100 mcg/L) were enrolled and randomly assigned (1:1) to receive either placebo or combination treatment consisting of intravenous iron, subcutaneous erythropoietin alpha, vitamin B12 and oral folic acid intake before elective cardiac surgery. The authors reported a significant reduction of RBC transfusions in previously treated patients but no differences were found among the two groups concerning length of stay, serious adverse events, post-operative complications, perioperative and 90-day mortality. Specifically, this is a great result in reducing administration of RBC transfusion, however, the real impact of this study should be seen from the economic point of view. In fact, even if cost-effective analyses between the two groups were difficult to estimate, the ratio between total and acquisition costs of administration of iron, vitamin B12 and erythropoietin was lower than RBC transfusions, favouring this short-term treatment in terms of reduction of hospital expense.
The results of the present study can be considered a starting point for future trials on the enhanced recovery after surgery (ERAS) pathways, also in the setting of thoracic surgery.
As already reported by several authors (5,6), ERAS protocols are associated with a shorter hospital length of stay, significant decrease in intensive care unit admission and a lower risk of cardio-pulmonary complications compared to patients receiving “classic” postop protocols. In this scenario, it is well-established the video-assisted thoracoscopic surgery (VATS) anatomical resections are associated with lower blood transfusion, shorter length of stay and lower incidence of cardiopulmonary complications when compared with open surgery for non-small cell lung cancer (NSCLC) (7-10).
The combination of ERAS pathways and the increased world-wide experience in VATS resections can expand the opportunities to treat more aggressive diseases (i.e., locally advanced NSCLC receiving induction therapy), giving the patients the chance to reduce postop complications and to recover faster. In this scenario, the preoperative management of anaemia can be an important issue to add to ERAS protocols, especially in those patients undergoing complex thoracic surgical procedures. In a recent analysis on 429 patients undergoing VATS lobectomy for NSCLC, Li et al. (11) demonstrated that an estimated intraoperative blood loss higher than 100 mL was a predictor for cardiopulmonary complications. In addition, another interesting issue is the relationship between RBC transfusion and survival in patients with NSCLC. Latif et al. (12) retrospectively analyzed 5,709 consecutive patients undergoing pulmonary resections for stage I–IIIa NSCLC and demonstrated a dose-response relationship with worse overall survival, disease free survival and recurrence by increasing unit of blood transfused. These results have been confirmed in other published series, confirming the prognostic role of RBC transfusions in thoracic surgery patients (13-20).
In the literature, several mechanisms of transfusion-related immunomodulation have been suggested, as decreased function of killer cells, decreased ratio of helper-to-suppressor T lymphocytes, reduced efficacy of antigen presentation, induced tolerance for specific antigens, suppression of hemopoiesis and depletion of extracellular arginine in the serum (21-23). Probably, the interactions between tumour microenvironment, immune cells and blood products (of RBC transfusions) may negatively modulate the host immune system, thus resulting in immune status imbalance, worse perioperative outcome and survival. In the perioperative setting, Nam et al. (24) reported that intraoperative RBC transfusions in patients with increased C-reactive protein level was significantly related to postop complications after cardiac surgery. On the other hand, our previous studies (25,26) on the relationship between immunity and thoracic surgery demonstrated that pre- and postoperative CRP levels may predict immediate and long-term mortality in all stages of operable NSCLC as well as in patients with resectable lung metastases. These results underline the need to “control” the host immune system, even after surgery, and to undertake anti-inflammatory pathways (i.e., reducing RBC transfusion) in order to avoid immune status imbalance and worse outcome. In the thoracic surgery setting, planning pre- and perioperative correction of anaemia (i.e., by administration of intravenous iron, subcutaneous erythropoietin alpha, vitamin B12 and oral folic acid) in those patients with high preoperative CRP level could allow to reduce post-operative complications and, in case of neoplasm, to improve survival. Some benefits would also be extended to those subsets of patients refusing RBC transfusions for religious or cultural reasons. Based on this data, we proposed a new scheme on this issue (regarding the preoperative and postoperative setting in major thoracic procedures) by adjusting the protocol by Muñoz et al. (Tables 1,2). We are aware that future randomized prospective trials must be performed to explore and confirm this proposal.
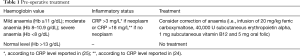
Full table

Full table
In conclusion, treatment of anaemia before surgery may play an important role in the setting of ERAS protocols, by further reducing hospital stay and improving recovering rate. According to the relationship between immune system and tumor cell suppression, the reduction of RBC transfusions may help not to alter host immunity and to reduce the risk of tumor progression. Future studies are needed to better understand this interesting issue.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Ranucci M, Baryshnikova E, Castelvecchio S, et al. Major bleeding, transfusions, and anaemia: the deadly triad of cardiac surgery. Ann Thorac Surg 2013;96:478-85. [Crossref] [PubMed]
- Muñoz M, Acheson AG, Bisbe E, et al. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia 2018;73:1418-31. [Crossref] [PubMed]
- Leahy MF, Hofmann A, Towler S, et al. Improved outcomes and reduced costs associated with a health-system-wide patient blood management program: a retrospective observational study in four major adult tertiary-care hospitals. Transfusion 2017;57:1347-58. [Crossref] [PubMed]
- Spahn DR, Schoenrath F, Spahn GH, et al. Effect of ultra-short-term treatment of patients with iron deficiency or anaemia undergoing cardiac surgery: a prospective randomised trial. Lancet 2019;393:2201-12. [Crossref] [PubMed]
- Medbery RL, Fernandez FG, Khullar OV. ERAS and patient reported outcomes in thoracic surgery: a review of current data. J Thorac Dis 2019;11:S976-86. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Yang CJ, Kumar A, Deng JZ, et al. A National Analysis of Short-term Outcomes and Long-term Survival Following Thoracoscopic Versus Open Lobectomy for Clinical Stage II Non-Small-Cell Lung Cancer. Ann Surg 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Laursen LØ, Petersen RH, Hansen HJ, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomy. Eur J Cardiothorac Surg 2016;49:870-5. [Crossref] [PubMed]
- Li S, Zhou K, Lai Y, et al. Estimated intraoperative blood loss correlates with postoperative cardiopulmonary complications and length of stay in patients undergoing video-assisted thoracoscopic lung cancer lobectomy: a retrospective cohort study. BMC Surg 2018;18:29. [Crossref] [PubMed]
- Latif MJ, Tan KS, Molena D, et al. Perioperative blood transfusion has a dose-dependent relationship with disease recurrence and survival in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;157:2469-77.e10. [Crossref] [PubMed]
- Nosotti M, Rebulla P, Riccardi D, et al. Correlation between perioperative blood transfusion and prognosis of patients subjected to surgery for stage I lung cancer. Chest 2003;124:102-7. [Crossref] [PubMed]
- Ng T, Ryder BA, Chern H, et al. Leukocyte-depleted blood transfusion is associated with decreased survival in resected early-stage lung cancer. J Thorac Cardiovasc Surg 2012;143:815-9. [Crossref] [PubMed]
- Moores DW, Piantadosi S, McKneally MF. Effect of perioperative blood transfusion on outcome in patients with surgically resected lung cancer. Ann Thorac Surg 1989;47:346-51. [Crossref] [PubMed]
- Piantadosi S, Moores DW, McKneally MF. The adverse effect of perioperative blood transfusion in lung cancer. Chest 1994;106:382S-4S. [Crossref] [PubMed]
- Wang T, Luo L, Huang H, et al. Perioperative blood transfusion is associated with worse clinical outcomes in resected lung cancer. Ann Thorac Surg 2014;97:1827-37. [Crossref] [PubMed]
- Pastorino U, Valente M, Cataldo I, et al. Perioperative blood transfusion and prognosis of resected stage Ia lung cancer. Eur J Cancer Clin Oncol 1986;22:1375-8. [Crossref] [PubMed]
- Thomas P, Michelet P, Barlesi F, et al. Impact of blood transfusions on outcome after pneumonectomy for thoracic malignancies. Eur Respir J 2007;29:565-70. [Crossref] [PubMed]
- Salter KD, Burt BM. Commentary: Postoperative blood transplantation for non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;157:2480-1. [Crossref] [PubMed]
- Hendrickson JE, Hillyer CD. Noninfectious serious hazards of transfusion. Anesth Analg 2009;108:759-69. [Crossref] [PubMed]
- Reddy SS, Leitman IM. Blood transfusions in the surgical patient: a gift of life, but at what cost? J Surg Res 2013;181:216-8. [Crossref] [PubMed]
- Procter LD, Meier CF, Hamilton C, et al. J Surg Res 2013;179:e183-7. [Crossref] [PubMed]
- Nam K, Jeon Y, Kim TK, et al. Intraoperative transfusion and an increased preoperative C-reactive protein level are associated with higher mortality after off-pump coronary artery bypass grafting. J Thorac Cardiovasc Surg 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Pastorino U, Morelli D, Leuzzi G, et al. Baseline and postoperative C-reactive protein levels predict mortality in operable lung cancer. Eur J Cancer 2017;79:90-7. [Crossref] [PubMed]
- Pastorino U, Morelli D, Leuzzi G, et al. Baseline and Postoperative C-reactive Protein Levels Predict Long-Term Survival After Lung Metastasectomy. Ann Surg Oncol 2019;26:869-75. [Crossref] [PubMed]


