The role for medical thoracoscopy in pneumothorax
Introduction
A pneumothorax is defined as an abnormal accumulation of air in the pleural space as first defined by Itard in 1803 after examination of necropsy specimens (1) (Figure 1). This potential space is bordered by the visceral pleura of the lung and the parietal pleura of the chest wall and normally contains only 0.2 to 0.5 mL of fluid (2). Although the intrapleural space is a negative pressure environment, air will not typically fill the space due to the relatively low sum of partial pressures of gases in the capillary bed. For the accumulation of air to occur one of three possible pathologies need to exist: (I) communication between the atmosphere and the pleural space; (II) communication between the alveolar and pleural spaces; or (III) organisms present in this normally sterile environment that produce gas as a byproduct of their metabolism (3).
Primary versus secondary spontaneous pneumothorax
Primary spontaneous pneumothoraces (PSP) are those that occur in people without underlying lung disease. Until the 2nd or 3rd decade of the 20th century it was commonly believed that spontaneous pneumothorax almost always indicated the presence of pulmonary tuberculosis (4). With increasing reports of spontaneous pneumothorax in people that had no evidence of tuberculosis, Kjaergaard first described the presence of spontaneous pneumothorax in healthy, young adult males in 1932 (5). PSP is now a known entity with a significant disease burden found more commonly in men and typically occurring in people in their 20’s. One study in the United Stated demonstrated an age-adjusted incidence of 7.4/100,000/year in men and 1.2/100,000/year in women (6). In the United Kingdom the overall rate of both primary and secondary pneumothorax presenting to their physicians was 24/100,000 for men and 9.8/100,000 for women. The mortality was 1.26 per million and 0.62 per million for men and women respectively (7). PSP most commonly presents when patients are not exerting themselves (8). The average recurrence rate for primary spontaneous pneumothorax with simple drainage is 30% but has been as high as 52% in some series (9).
In addition to being a young male, smoking appears to be a significant risk factor for the development of PSP. Tobacco smoking may increase the risk of PSP by 22 times in men and 9 times in women (10). Also, the inhalation of cannabis and cocaine smoke has been demonstrated to be risk factors for PSP (11,12).
The etiology of PSP remains uncertain. Classically described as owing to the rupture of bullae or “blebs,” the demonstration by Janssen et al. that those with a first episode of pneumothorax had no more anatomic abnormalities than those with recurrent pneumothoraces has lent doubt that bullae may be a major precipitating factor (13). Recent evidence with autofluorescent pleuroscopy does, however, suggest that some anatomic abnormality is a key-instigating factor (14). Associated medical conditions may include Marfan syndrome, Burg-Hogge-Dube syndrome, thoracic endometriosis, and homocysteinuria.
Secondary spontaneous pneumothoraces (SSP) are associated with an underlying medical condition. Although the recurrence rate is similar to PSP, mortality is higher due to the underlying pulmonary pathology and consequently decreased pulmonary reserve (15). Historically tuberculosis was the most common cause, however, more recently chronic obstructive pulmonary disease (COPD) is being cited as the most frequently associated lung disease with SSPs in 57% to 70% in some series (16,17). In age-matched controls with COPD each pneumothorax occurrence increases the mortality by 3.5 times (18). Other conditions that lead to secondary pneumothorax include cystic fibrosis, Pneumocystis jirovecii pneumonia, necrotizing pneumonia, lymphangioleiomyomatosis, sarcoidosis, idiopathic pulmonary fibrosis, histiocytosis X, connective tissue diseases, and thoracic malignancies (19).
Laennec described the presenting symptoms and signs of this condition in the early 19th century (20). Patients may complain of chest pain and/or dyspnea. Those patients that develop SSP tend to be more symptomatic than those that are afflicted with PSP (15,21). Additionally those patients with SSP may have breathlessness that is out of proportion to the size of the pneumothorax (22). In some cases a pneumothorax can cause hypoxia and hemodynamic instability and may require emergent intervention.
Staging of pneumothorax
The size and stage of pneumothoraces are defined by the radiographic and thoracoscopic appearance of the pleural cavity, respectively. The size is classically estimated by the posterior-anterior (PA) chest radiograph by taking the ratio of the hemithorax volume (cubed) minus the lung volume (cubed) divided by the hemithorax volume (cubed) (23).
% Pneumothorax =100[1– diameter lung3/diameter hemithorax3] (24).
Computed tomography (CT) scanning is now, however, regarded as the best means of establishing the size of a pneumothorax and has been calibrated in a lung model experiment (25,26).
In 1981 Vanderschueren defined four stages of pneumothorax based upon direct visualization of the pleural space: Stage I, lung endoscopically normal; Stage II, pleuro-pulmonary adhesions; Stage III, small bullae and blebs <2 cm in diameter; Stage IV, large bullae >2 cm in diameter (27). Although this would imply a correlation between the pathophysiology of spontaneous pneumothorax and the appearance of blebs thus far there has been no definitive data to support this theory (28).
Evolution of medical thoracoscopy
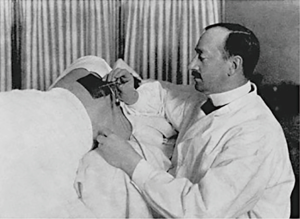
The pleural cavity was first explored in 1866 when an urologist by the name of Francis Richard Cruse used a cystoscope through a thoracocutaneous fistula in a girl suffering from a chronic empyema (29). It was not until almost four decades later, however, that the true clinical application of thoracoscopy was realized. Hans Christian Jacobaeus was a Swedish internist who is widely recognized as the true innovator of thoracoscopy. His first paper describing the technique of pleural cavity examination in two patients with pleurisy using a cystoscope was published in 1910 (30) (Figure 2). This early success was soon followed by several case series describing his technique on a variety of conditions to include pneumothorax (31). His continued innovations and willingness to collaborate, as well as, the use of thoracoscopy in collapse therapy for pulmonary tuberculosis led to the widespread use of this technique in a manner very similar to medical thoracoscopy in the present day (32). The introduction of antituberculous drugs made collapse therapy obsolete, however, physicians knowledgeable in the “Jacobaeus Operation” began to apply the art of thoracoscopy to a variety of pleural diseases (33).
In the 1990’s surgeons began to apply techniques learned from laparoscopic cholecystectomy to procedures performed in the thorax. The term video-assisted thoracoscopic surgery (VATS) is used to describe those thoracoscopic procedures performed exclusively by thoracic surgeons. The VATS procedure differs from medical thoracoscopy in the access to the chest cavity and the ability to perform such surgical interventions as stapler resection, bullectomy, lobectomy, and pneumonectomy (32). Additionally, unlike medical thoracoscopy, which can by done under local anesthesia in an endoscopy suite, VATS requires an operating room and general anesthesia with double lumen tube intubation. Although medical thoracoscopy is limited in the interventions that can be performed, for many indications medical thoracoscopy is more cost effective than VATS and can be performed in patients that would otherwise be poor candidates for surgical intervention due to underlying comorbidities (34).
Medical thoracoscopy has been traditionally performed with rigid instruments through one or two ports inserted into the lateral chest wall as described originally by Jacobaeus. The facility of the procedure is enhanced by the fact that it can typically be done in patients using only local anesthesia and conscious sedation in an endoscopy suite (35). In the 1970’s a flexible bronchoscope was first used to perform medical thoracoscopy (36). The lack of familiarity with rigid instruments by many pulmonary physicians made this an attractive option, however, the flexibility of the bronchoscope made it more difficult to control in the pleural space especially when performing pleural biopsies (37).
In 1998 a hybrid or “semiflexible” thoracoscope was developed. This scope utilized the fiberoptic technology developed by Shigeto Ikeda for flexible bronchoscopes and combined a 50 cm rigid proximal section with a flexible tip that had a 60° up and 130° down angulation (38). The similar operating features of this instrument to flexible bronchoscopy have led to more widespread use of medical thoracoscopy by pulmonary physicians.
Diagnostic capability of medical thoracoscopy
The diagnostic capability for a variety of pleural diseases by medical thoracoscopy has been well established. One of the primary uses of medical thoracoscopy has been in the diagnosis and treatment of malignant pleural effusions. Rigid thoracoscopy has been demonstrated to have a diagnostic rate for pleural effusion greater than 93% when compared to pleural fluid cytology and/or closed pleural biopsy (39-41). Studies involving the semiflexible thoracoscope are comparable to those of the rigid scope despite the smaller biopsy size obtained by the semiflexible forceps. The original report of the semiflexible thoracoscope reported a sensitivity of 81% compared to 62% for an Abrams needle in undiagnosed pleural effusion (38). As physicians have gained more experience with this instrument the diagnostic yields have rivaled those of the rigid thoracoscope in pleural effusions with rates reported to be greater than 90% (42-44).
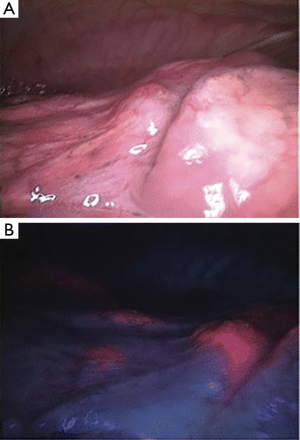
Newer technologies have enhanced the diagnostic capability of medical thoracoscopy. A small study in patients with PSP compared to controls was done with fluorescein-enhanced autofluorescence thoracoscopy (FEAT). The autofluorescence (AF) unit uses a blue light under which fluorescein will emit a yellow-green light. These subjects inhaled an aerosolized 10% fluorescein solution and then underwent thoracoscopy using the DAFE AF endoscopy unit. Extensive subpleural accumulation and fluorescein leakage corresponding to high-grade lesions was seen in PSP patients that were not seen in controls. Interestingly these lesions did not necessarily correspond to bleb, bullae or other lesions visualized under white light thoracoscopy (14) (Figure 3). Narrow band imaging uses two narrow bands of light that are absorbed by hemoglobin and when applied in the gastrointestinal or respiratory tract will demonstrate abnormal vascular patterns that correspond to areas of malignant epithelium increasing diagnostic accuracy. This technology has been applied to medical thoracoscopy increasing the diagnostic accuracy for pleural malignancy over that of white light thoracoscopy (45).
Therapeutic capability for pneumothorax
Both the British Thoracic Society (BTS) and the American College of Chest Physicians (ACCP) have published guidelines regarding the management of spontaneous and secondary pneumothorax. For the first occurrence of PSP greater than 2 to 3 cm the accepted approach is to perform simple drainage with small-bore chest tube (15,21). The treatment for recurrence is less clear with simple aspiration being unsuccessful in 15-62% (46). Chemical pleurodesis is superior to simple tube drainage for prevention of recurrence. One study compared the treatment of PSP with simple aspiration (SA) versus pleurodesis with talc or tetracycline. Those patients who underwent SA had a recurrence rate of 36% whereas those patients treated with chemical pleurodesis had a recurrence rate of 8-13% (47). Talc is the preferred agent and is superior to other agents. There were some concerns regarding the development of acute respiratory distress syndrome (ARDS) following talc pleurodesis, particularly in the United States where small particle talc was being utilized. Further evaluation using talc particles >10 microns has demonstrated safety, however (48). For SSP the general consensus is that once the patient has been medically stabilized with chest drainage that a thoracoscopic or open surgical procedure should be offered over sclerosant instillation via a chest tube due to the higher recurrence rates in the latter group and the significant mortality associated with recurrence after a first SSP (Figure 4). The exact surgical method for treating pneumothorax is controversial due to the lack of good prospective trials comparing various methods but the recent recommendations from the BTS suggest that a surgical option should be obtained for: second ipsilateral pneumothorax, first contralateral pneumothorax, synchronous bilateral spontaneous pneumothorax, persistent air leak (despite 5 to 7 days of chest tube drainage) or failure of lung re-expansion, spontaneous hemothorax, and professionals at risk (e.g., pilots, divers), pregnancy (15). Currently the authors of the ACCP guidelines state that either medical or surgical thoracoscopy is acceptable (21). In the largest studies on pneumothorax management, talc pleurodesis via thoracoscopy is safe, effective at preventing recurrence and cost-effective (49).
There does not appear to be good adherence to the current guidelines. This may be due to some of the differences between the ACCP and BTS guidelines as well as the lack of good data to help guide management decisions (50). Some have advocated a simple stepwise approach to first occurrence of PSP management to include SA, followed by tube thoracostomy, and then surgical options should the more conservative approach fail (51). Again, the exact method for further surgical options could not be recommended at this time and will be due to physician preference and training, patient selection, and institutional practice.
Video-assisted thoracoscopy has been described as the treatment of choice for pneumothorax with a very low recurrence rate (52). VATS gives the option to perform bullectomy, apical pleurectomy, mechanical abrasion and can be converted over to open thoracotomy if needed. However, there is no convincing data that bullectomy will decrease the rate of recurrent pneumothorax and there is controversy regarding the role that bullae play in both initial and recurrent spontaneous pneumothorax. A study performed that compared thoracoscopic findings in initial episodes to recurrent pneumothoraces found no difference in the number bullae (13). Electromicrographic studies of resected bullae have also failed to definitively demonstrate locations of air leaks within the bullae (53). As such some have stated that the presence of bullae or blebs in cases of spontaneous pneumothorax has no implication on recurrence and should not dictate therapeutic interventions (54).
Medical thoracoscopy under local anesthesia has been demonstrated to be safe and effective. In the 1970’s there were reports of intervention of pneumothorax using high frequency electrocoagulation therapy (single-photon emission computed tomography, SPECT) with a rigid thoracoscope. The recurrence rate for pneumothorax, however, was as high at 18% (55). SPECT has also been used with a fiberoptic bronchoscope coupled with the outer irrigation sheath of a hysteroscope in the pleural cavity. Successful therapy was achieved in 80% of patients, but 10% of the patients in the series required open thoracotomy (56). In patients with bullae <2 cm pleurodesis with medical thoracoscopy and talc poudrage has been shown to be 95% effective (57). The European Study on Medical Video-Assisted Thoracoscopy compared the recurrence rate for PSP for patients treated with SA versus VATS with talc pleurodesis. Those that underwent VATS only had a recurrence rate of 5% compared to 34% in the SA group (58). Although the procedure used in this study was called a VATS, it was actually performed as a medical thoracoscopy in sedated patients rather than the procedure under general anesthesia done by thoracic surgeons typically referred to as VATS. The long-term safety and efficacy of medical thoracoscopy and talc poudrage has been demonstrated in a single center study with a median follow-up of greater than 10 years. Again the rate of recurrence was shown to be comparative to VATS at 5% in nonsmoking patients with blebs <2 cm and did not have a significant detrimental effect on long-term lung function (59).
In patients with Vanderschueren’s stage III or IV blebs/bullae, some advocate that VATS should be the preferred modality over medical thoracoscopy in part because of the ability to perform bullectomy or apical pleurectomy (Figure 5). There is not good evidence that bullectomy will prevent pneumothorax recurrence, but pleurodesis has demonstrated to be very effective. Pleurodesis via VATS is as effective as apical pleurectomy but with less morbidity (60). Patients with COPD are at significant risk for developing pneumothorax and because of their disease and comorbid illnesses may be very poor candidates for surgical procedures with general anesthesia, paralytics, and double lumen endotracheal tubes. However, without intervention these individuals are at a significant risk of death (61). A study of 41 patients with COPD who had an average forced expiratory volume in 1 sec (FEV1) of 41% of predicted and of which two thirds were ASA grade 4 was performed with medical thoracoscopy and talc poudrage. Of note is that 44% of the patients had Vanderscheuren’s stage III or IV blebs/bullae. The reported success rate was 95% at a median follow-up of 35 months (62). There was a 10% mortality rate in this study that occurred in four patients with FEV1 <40% predicted and ischemic heart disease. This is in comparison for reports of overall mortality rates of <1% for VATS in the elderly (63). The authors point out that unlike the VATS study where the FEV1 was >1.6 L, the patients in their study had a median FEV1 of 0.58 L, however, they caution against the use of medial thoracoscopy with talc poudrage in COPD patients with FEV1 <0.7 L and concomitant ischemic heart disease (62). Although the numbers are small, this study suggests that medical thoracoscopy with talc pleurodesis is effective in secondary pneumothorax due to COPD even in patients with large bullae/blebs.
In patients who have had a previous medical thoracoscopy there is concern about repeating a thoracoscopy on the same side due to the possibility of adhesions interfering with visualization or increasing the risk of complications. Repeat medical thoracoscopy has been shown to be feasible and safe in a small series of 29 patients. This includes patients that have had a prior talc pleurodesis. Additionally most of the patients were able to undergo a successful talc pleurodesis during their subsequent thoracoscopy (64). This compares to another small series of 39 patients that underwent VATS following a previous talc poudrage on the ipsilateral side where the procedure was successful in nearly 70% of patients (65).
Ultrasound is becoming a very important tool in the field of pulmonary medicine and is an important adjunct in decreasing complications of procedures such as thoracentesis, including in those patients that have a coagulopathy (66,67). Thoracic ultrasound has been used with both rigid and semi-flexible medical thoracoscopy and has been cited as allowing for safe thoracoscopy in patients with pleural adhesions (68). Additionally when compared to controls thoracic ultrasonography significantly decreases the rate of failed pleural access in the investigation of pleural exudates (69-83).
Conclusions
Pneumothorax is commonly encountered by physicians that care for diseases of the thorax and can be a life-threatening condition. For most initial cases of PSP simple aspiration is therapeutic although has a significant recurrence rate. For most cases of SSP and recurrences of PSP there is little debate that additional intervention above simple aspiration with or without tube thoracostomy should be done. There is widespread discussion, however, on the exact intervention that should be performed in these circumstances. Although VATS has been stated to be the gold-standard intervention, in many cases medical thoracoscopy with pleurodesis will achieve similar therapeutic success with comparative recurrence rates. Additionally the advent of the semi-flexible pleuroscope has led to increased experience in medical thoracoscopy by pulmonologists. The role of medical thoracoscopy in the diagnosis and treatment of pleural disease is as relevant today as it was when Jacobaeus pioneered this important procedure. The community of thoracic physicians can continue to advance the applications of medical thoracoscopy by utilizing the collaborative spirit that Jacobaeus demonstrated over a century ago.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Itard JE. Dissertation sur le pneumothorax ou les congestions gaseuses quise forment dans la poitrine. Thesis, Paris, 1803.
- Jantz MA, Antony VB. Pathophysiology of the pleura. Respiration 2008;75:121-33. [PubMed]
- Noppen M, De Keukeleire T. Pneumothorax. Respiration 2008;76:121-7. [PubMed]
- MYERS JA. Simple spontaneous pneumothorax. Dis Chest 1954;26:420-41. [PubMed]
- Kjaergaard H. Spontanous Pneumothorax in the Apparently Healthy. Copenhagen, Levine and Munksgaard. Acta Med Scand 1932;43:1-159.
- Melton LJ 3rd, Hepper NG, Offord KP. Incidence of spontaneous pneumothorax in Olmsted County, Minnesota: 1950 to 1974. Am Rev Respir Dis 1979;120:1379-82. [PubMed]
- Gupta D, Hansell A, Nichols T, et al. Epidemiology of pneumothorax in England. Thorax 2000;55:666-71. [PubMed]
- Bense L, Wiman LG, Hedenstierna G. Onset of symptoms in spontaneous pneumothorax: correlations to physical activity. Bense L1, Wiman LG, Hedenstierna G. Eur J Respir Dis 1987;71:181-6. [PubMed]
- Schramel FM, Postmus PE, Vanderschueren RG. Current aspects of spontaneous pneumothorax. Eur Respir J 1997;10:1372-9. [PubMed]
- Bense L, Eklund G, Wiman LG. Smoking and the increased risk of contracting spontaneous pneumothorax. Chest 1987;92:1009-12. [PubMed]
- Gill A. Bong lung: regular smokers of cannabis show relatively distinctive histologic changes that predispose to pneumothorax. Am J Surg Pathol 2005;29:980-2. [PubMed]
- Devlin RJ, Henry JA. Clinical review: Major consequences of illicit drug consumption. Crit Care 2008;12:202. [PubMed]
- Janssen JP, Schramel FM, Sutedja TG, et al. Videothoracoscopic appearance of first and recurrent pneumothorax. Chest 1995;108:330-4. [PubMed]
- Noppen M, Dekeukeleire T, Hanon S, et al. Fluorescein-enhanced autofluorescence thoracoscopy in patients with primary spontaneous pneumothorax and normal subjects. Am J Respir Crit Care Med 2006;174:26-30. [PubMed]
- MacDuff A, Arnold A, Harvey J, et al. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii18-31. [PubMed]
- Chen CH, Liao WC, Liu YH, et al. Secondary spontaneous pneumothorax: which associated conditions benefit from pigtail catheter treatment? Am J Emerg Med 2012;30:45-50. [PubMed]
- Guo Y, Xie C, Rodriguez RM, et al. Factors related to recurrence of spontaneous pneumothorax. Respirology 2005;10:378-84. [PubMed]
- Dines DE, Clagett OT, Payne WS. Spontaneous pneumothorax in emphysema. Mayo Clin Proc 1970;45:481-7. [PubMed]
- Tschopp JM, Rami-Porta R, Noppen M, et al. Management of spontaneous pneumothorax: state of the art. Eur Respir J 2006;28:637-50. [PubMed]
- Laennec RTH. eds. De l’Auscultation Médiate ou Traité du Diagnostic des Maladies des Poumons et du Coeur. Paris: Brosson & Chaudé, 1819.
- Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001;119:590-602. [PubMed]
- Tanaka F, Itoh M, Esaki H, et al. Secondary spontaneous pneumothorax. Ann Thorac Surg 1993;55:372-6. [PubMed]
- Henry M, Arnold T, Harvey J, et al. BTS guidelines for the management of spontaneous pneumothorax. Thorax 2003;58 Suppl 2:ii39-52.[PubMed]
- Light RW. Pneumothorax. In: Light RW. eds. Pleural Diseases, Fifth Edition. Philadelphia: Wolters Kluwer, 2007:306-39.
- Archer GJ, Hamilton AA, Upadhyay R, et al. Results of simple aspiration of pneumothoraces. Br J Dis Chest 1985;79:177-82. [PubMed]
- Ng AW, Chan KW, Lee SK. Simple aspiration of pneumothorax. Singapore Med J 1994;35:50-2. [PubMed]
- Vanderschueren RG. Pleural talcage in patients with spontaneous pneumothorax (author’s transl). Poumon Coeur 1981;37:273-6. [PubMed]
- Tschopp JM, Frey JG. Treatment of primary spontaneous pneumothorax by simple talcage under medical thoracoscopy. Monaldi Arch Chest Dis 2002;57:88-92. [PubMed]
- Gordon S. Clinical reports of rare cases, occurring in the Whitworth and Hardwicke Hospitals: most extensive pleuritic effusion rapidly becoming purulent, paracentesis, introduction of a drainage tube, recovery, examination of interior of pleura by the endoscope. Dublin Quarterly J Med Sci 1866;41:83-90.
- Jacobaeus HC. Über die Möglichkeit die Zystoskopie bei Untersuchung seröser Höhlungen anzuwenden. Münch Med Woch 1910;57:2090-2.
- Jacobaeus HC. Kurze Übersicht über meine Erfahrungen der Laparo-Thorakoskopie. Münch Med Woch 1911;58:2017-9.
- Marchetti GP, Pinelli V, Tassi GF. 100 years of thoracoscopy: historical notes. Respiration 2011;82:187-92. [PubMed]
- Loddenkemper R. Thoracoscopy--state of the art. Eur Respir J 1998;11:213-21. [PubMed]
- Tassi GF, Tschopp JM. The centenary of medical thoracoscopy. Eur Respir J 2010;36:1229-31. [PubMed]
- Froudarakis ME, Noppen M. Medical thoracoscopy: new tricks for an old trade. Respiration 2009;78:373-4. [PubMed]
- Senno A, Moallem S, Quijano ER, et al. Thoracoscopy with the fiberoptic bronchoscope. A simple method in diagnosing pleuropulmonary diseases. J Thorac Cardiovasc Surg 1974;67:606-11. [PubMed]
- Davidson AC, George RJ, Sheldon CD, et al. Thoracoscopy: assessment of a physician service and comparison of a flexible bronchoscope used as a thoracoscope with a rigid thoracoscope. Thorax 1988;43:327-32. [PubMed]
- McLean AN, Bicknell SR, McAlpine LG, et al. Investigation of pleural effusion: an evaluation of the new Olympus LTF semiflexible thoracofiberscope and comparison with Abram’s needle biopsy. Chest 1998;114:150-3. [PubMed]
- Boutin C, Astoul P. Diagnostic thoracoscopy. Clin Chest Med 1998;19:295-309. [PubMed]
- Colt HG. Thoracoscopy. A prospective study of safety and outcome. Chest 1995;108:324-9. [PubMed]
- Blanc FX, Atassi K, Bignon J, et al. Diagnostic value of medical thoracoscopy in pleural disease: a 6-year retrospective study. Chest 2002;121:1677-83. [PubMed]
- Munavvar M, Khan MA, Edwards J, et al. The autoclavable semirigid thoracoscope: the way forward in pleural disease? Eur Respir J 2007;29:571-4. [PubMed]
- Wang Z, Tong ZH, Li HJ, et al. Semi-rigid thoracoscopy for undiagnosed exudative pleural effusions: a comparative study. Chin Med J (Engl) 2008;121:1384-9. [PubMed]
- Lee P, Colt HG. Rigid and semirigid pleuroscopy: the future is bright. Respirology 2005;10:418-25. [PubMed]
- Ishida A, Ishikawa F, Nakamura M, et al. Narrow band imaging applied to pleuroscopy for the assessment of vascular patterns of the pleura. Respiration 2009;78:432-9. [PubMed]
- Chen JS, Hsu HH, Tsai KT, et al. Salvage for unsuccessful aspiration of primary pneumothorax: thoracoscopic surgery or chest tube drainage? Ann Thorac Surg 2008;85:1908-13. [PubMed]
- Almind M, Lange P, Viskum K. Spontaneous pneumothorax: comparison of simple drainage, talc pleurodesis, and tetracycline pleurodesis. Thorax 1989;44:627-30. [PubMed]
- Bridevaux PO, Tschopp JM, Cardillo G, et al. Short-term safety of thoracoscopic talc pleurodesis for recurrent primary spontaneous pneumothorax: a prospective European multicentre study. Eur Respir J 2011;38:770-3. [PubMed]
- Tschopp JM, Schnyder JM, Froudarakis M, et al. VATS or simple talc poudrage under medical thoracoscopy for recurrent spontaneous pneumothorax. Eur Respir J 2009;33:442-3. [PubMed]
- Elsayed H, Kent W, McShane J, et al. Treatment of pneumothoraces at a tertiary centre: are we following the current guidelines? Interact Cardiovasc Thorac Surg 2011;12:430-3. [PubMed]
- Kaneda H, Nakano T, Taniguchi Y, et al. Three-step management of pneumothorax: time for a re-think on initial management. Interact Cardiovasc Thorac Surg 2013;16:186-92. [PubMed]
- Cardillo G, Facciolo F, Giunti R, et al. Videothoracoscopic treatment of primary spontaneous pneumothorax: a 6-year experience. Ann Thorac Surg 2000;69:357-61; discussion 361-2. [PubMed]
- Ohata M, Suzuki H. Pathogenesis of spontaneous pneumothorax. With special reference to the ultrastructure of emphysematous bullae. Chest 1980;77:771-6. [PubMed]
- Schramel FM, Zanen P. Blebs and/or bullae are of no importance and have no predictive value for recurrences in patients with primary spontaneous pneumothorax. Chest 2001;119:1976-7. [PubMed]
- Tschopp JM, Brutsche M, Frey JG. Treatment of complicated spontaneous pneumothorax by simple talc pleurodesis under thoracoscopy and local anaesthesia. Thorax 1997;52:329-32. [PubMed]
- Tsukamoto T, Nakamura H, Satoh T, et al. Comparative studies using a rigid thoracoscope and fiberoptic bronchoscope to treat spontaneous pneumothorax. Chest 1991;100:953-8. [PubMed]
- Tschopp JM, Brutsche M, Frey JG. Treatment of complicated spontaneous pneumothorax by simple talc pleurodesis under thoracoscopy and local anaesthesia. Thorax 1997;52:329-32. [PubMed]
- Tschopp JM, Boutin C, Astoul P, et al. Talcage by medical thoracoscopy for primary spontaneous pneumothorax is more cost-effective than drainage: a randomised study. Eur Respir J 2002;20:1003-9. [PubMed]
- Györik S, Erni S, Studler U, et al. Long-term follow-up of thoracoscopic talc pleurodesis for primary spontaneous pneumothorax. Eur Respir J 2007;29:757-60. [PubMed]
- Rena O, Massera F, Papalia E, et al. Surgical pleurodesis for Vanderschueren's stage III primary spontaneous pneumothorax. Eur Respir J 2008;31:837-41. [PubMed]
- Videm V, Pillgram-Larsen J, Ellingsen O, et al. Spontaneous pneumothorax in chronic obstructive pulmonary disease: complications, treatment and recurrences. Eur J Respir Dis 1987;71:365-71. [PubMed]
- Lee P, Yap WS, Pek WY, et al. An Audit of medical thoracoscopy and talc poudrage for pneumothorax prevention in advanced COPD. Chest 2004;125:1315-20. [PubMed]
- Jaklitsch MT, DeCamp MM Jr, Liptay MJ, et al. Video-assisted thoracic surgery in the elderly. A review of 307 cases. Chest 1996;110:751-8. [PubMed]
- Breen D, Fraticelli A, Greillier L, et al. Redo medical thoracoscopy is feasible in patients with pleural diseases - a series. Interact Cardiovasc Thorac Surg 2009;8:330-3. [PubMed]
- Doddoli C, Barlési F, Fraticelli A, et al. Video-assisted thoracoscopic management of recurrent primary spontaneous pneumothorax after prior talc pleurodesis: a feasible, safe and efficient treatment option. Eur J Cardiothorac Surg 2004;26:889-92. [PubMed]
- Grogan DR, Irwin RS, Channick R, et al. Complications associated with thoracentesis. A prospective, randomized study comparing three different methods. Arch Intern Med 1990;150:873-7. [PubMed]
- Patel MD, Joshi SD. Abnormal preprocedural international normalized ratio and platelet counts are not associated with increased bleeding complications after ultrasound-guided thoracentesis. AJR Am J Roentgenol 2011;197:W164-8. [PubMed]
- Hersh CP, Feller-Kopman D, Wahidi M, et al. Ultrasound guidance for medical thoracoscopy: a novel approach. Respiration 2003;70:299-301. [PubMed]
- Medford AR, Agrawal S, Bennett JA, et al. Thoracic ultrasound prior to medical thoracoscopy improves pleural access and predicts fibrous septation. Respirology 2010;15:804-8. [PubMed]
- Boskovic T, Stanic J, Pena-Karan S, et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis 2014;6:S99-107. [PubMed]
- Tsakiridis K, Mpakas A, Kesisis G, et al. Lung inflammatory response syndrome after cardiac-operations and treatment of lornoxicam. J Thorac Dis 2014;6:S78-98. [PubMed]
- Tsakiridis K, Zarogoulidis P, Vretzkakis G, et al. Effect of lornoxicam in lung inflammatory response syndrome after operations for cardiac surgery with cardiopulmonary bypass. J Thorac Dis 2014;6:S7-20. [PubMed]
- Argiriou M, Kolokotron SM, Sakellaridis T, et al. Right heart failure post left ventricular assist device implantation. J Thorac Dis 2014;6:S52-9. [PubMed]
- Madesis A, Tsakiridis K, Zarogoulidis P, et al. Review of mitral valve insufficiency: repair or replacement. J Thorac Dis 2014;6:S39-51. [PubMed]
- Siminelakis S, Kakourou A, Batistatou A, et al. Thirteen years follow-up of heart myxoma operated patients: what is the appropriate surgical technique? J Thorac Dis 2014;6:S32-8. [PubMed]
- Foroulis CN, Kleontas A, Karatzopoulos A, et al. Early reoperation performed for the management of complications in patients undergoing general thoracic surgical procedures. J Thorac Dis 2014;6:S21-31. [PubMed]
- Nikolaos P, Vasilios L, Efstratios K, et al. Therapeutic modalities for Pancoast tumors. J Thorac Dis 2014;6:S180-93. [PubMed]
- Koutentakis M, Siminelakis S, Korantzopoulos P, et al. Surgical management of cardiac implantable electronic device infections. J Thorac Dis 2014;6:S173-9. [PubMed]
- Spyratos D, Zarogoulidis P, Porpodis K, et al. Preoperative evaluation for lung cancer resection. J Thorac Dis 2014;6:S162-6. [PubMed]
- Porpodis K, Zarogoulidis P, Spyratos D, et al. Pneumothorax and asthma. J Thorac Dis 2014;6:S152-61. [PubMed]
- Panagopoulos N, Leivaditis V, Koletsis E, et al. Pancoast tumors: characteristics and preoperative assessment. J Thorac Dis 2014;6:S108-15. [PubMed]
- Visouli AN, Darwiche K, Mpakas A, et al. Catamenial pneumothorax: a rare entity? Report of 5 cases and review of the literature. J Thorac Dis 2012;4:17-31. [PubMed]
- Zarogoulidis P, Chatzaki E, Hohenforst-Schmidt W, et al. Management of malignant pleural effusion by suicide gene therapy in advanced stage lung cancer: a case series and literature review. Cancer Gene Ther 2012;19:593-600. [PubMed]