
Patient outcomes post-pulmonary resection for synchronous bone-metastatic non-small cell lung cancer
Introduction
Although the standard treatment for stage IV non-small cell lung cancer (NSCLC) is chemotherapy, surgeons have performed curative resection in patients with NSCLC who present with oligometastases, especially with synchronous solitary brain or adrenal metastasis (1-4). Endo and colleagues (5) reported that the five-year overall survival (OS) for clinical T1-2N0-1 lung cancer patients with a single-organ metastatic lesion who underwent complete resection of both the primary and metastatic tumors was 44.7%. Loi and colleagues (6) reported that the five-year OS of NSCLC patients with synchronous isolated brain and/or adrenal metastases who underwent locally ablative treatments (surgery and/or radiotherapy) for both the primary lung tumor and distant metastases was 34.4%. However, few reports describing the surgical resection of the primary lung tumor in NSCLC patients with isolated bone metastases exist, and the indication for surgical treatment in this population is controversial (7-9).
The median OS of lung cancer patients with bone metastases is reportedly 6–13 months, while their two-year OS rates are approximately 13–14% (10-12). Patrini and colleagues (13) suggested that bone metastasis cannot be considered an oligometastatic condition in any situation because of worse outcomes after surgical treatment (9,14). On the other hand, Bates and colleagues (12) reported that patients with limited metastatic NSCLC to the bone have improved OS relative to all other M1b lung tumors with lung, liver, or brain metastases. De Felice and colleagues (15) indicated that radiotherapy can reduce the volumes of metastatic bone lesions, and it was also reported that favorable survival rates can be expected in patients with oligometastatic NSCLC who achieve complete resection of their primary tumor combined with radical control of their distant lesions (8).
Herein, we report the outcomes of curative-intent surgical pulmonary resection in NSCLC patients with synchronous isolated bone metastases, and review the positive predictors of OS and progression-free survival (PFS) in such patients who underwent radical treatments for both the primary lung tumor and bone metastases.
Methods
Patients
We retrospectively reviewed the medical records of 236 patients with lung cancer who had bone metastases and who underwent treatment of any kind between January 2008 and December 2013 at the National Hospital Organization, Hokkaido Cancer Center, Japan. After excluding those with metastases in organs other than the bone, those with metachronous bone metastasis (i.e., no bone metastasis was present when the lung cancer was first diagnosed), those with small cell lung cancer histology, and those who underwent first-line palliative treatment, 41 patients (17.4%) who had synchronous isolated bone-metastatic NSCLC that was managed with radical treatments (surgery and/or chemotherapy and/or radiotherapy) for both the primary lung tumor and bone metastases were included in this study (Figure 1). Nine of these 41 patients underwent curative-intent resection of the primary pulmonary lesion. Pretreatment evaluation included chest and upper abdominal computed tomography (CT), brain magnetic resonance imaging, whole-body positron emission tomography-CT, and/or 99mTc-bone scintigraphy. The tumors were classified according to the World Health Organization classification for cell types upon histological analysis (16). The clinical or pathological disease stages were defined based on the general rules of the TNM Classification of Malignant Tumors (7th edition) (17).
Treatments
Radical treatments included surgical resection of the primary lung tumor, systemic cytotoxic chemotherapy and/or tyrosine kinase inhibitor (TKI) therapy, and/or surgical resection and/or radiotherapy for bone metastases. Systemic cytotoxic chemotherapy and/or TKI therapy were standard treatment(s) for patients with stage IV NSCLC according to the Guidelines for Diagnosis and Treatment of the Lung Cancer established by The Japan Lung Cancer Society. Curative-intent surgical resection of the primary lung tumor involved lobectomy as well as systematic hilar and mediastinal lymph node dissection, which was the standard procedure for primary lung tumors at our institute; patients who underwent pulmonary wedge resection and segmentectomy were excluded from the study. One patient who was excluded underwent wedge resection to diagnose a lung metastasis and subsequently underwent first-line palliative treatment, but died 10 months later; furthermore, another patient who was excluded because of missing data also underwent wedge resection to diagnose a lung lesion. The radiotherapy doses for metastatic bone lesions were between 25 Gy in five fractions to 50 Gy in 25 fractions.
Statistical analysis
The Mann-Whitney U-test and Chi-square test were used to compare continuous and categorial variables, respectively. The Kaplan-Meier method was used to determine the OS, PFS, and disease-free survival (DFS). OS was defined as the interval between the date of treatment initiation and that of death from any cause. PFS was defined as the period spanning and following any treatment for cancer until disease progression or death from any cause. DFS was defined as the duration between anticancer treatment completion and disease recurrence or death from any cause other than cancer. The comparisons of predicted survival were performed using the log-rank test and Cox regression analysis, with a P value <0.05 considered statistically significant. Variables shown to be significantly associated with survival on univariate analysis were entered into a Cox multivariate analysis model to assess their independent influences. All statistical analyses were performed using the SPSS software version 22.0 (IBM Corp., Armonk, NY, USA).
Ethical approval
This retrospective study was approved by the National Hospital Organization, Hokkaido Cancer Center Institutional Review Board (approval number: 302-167); the requirement to obtain informed consent was waived.
Results
Patients’ characteristics and treatment
Forty-one patients with synchronous isolated bone-metastatic NSCLC underwent radical treatment for both the primary lung tumor and bone metastases between January 2008 and December 2013 at the National Hospital Organization, Hokkaido Cancer Center, Japan. The characteristics of these patients are summarized in Table 1. The rate of clinical N0–1 disease among the 9 patients who underwent resection of the primary pulmonary tumor (100%) was significantly higher than that among the 32 patients who did not undergo this procedure (34.4%). Furthermore, all patients who underwent primary lung tumor resection (100%) were administered radiotherapy for metastatic bone lesions, whereas a significantly lower proportion (56.3%) of the 32 patients who did not undergo primary lung tumor resection received the same. There were no significant differences between patients who underwent resection of the primary lung tumor and those who did not in terms of median age, sex, Eastern Cooperative Oncology Group (ECOG) performance status (PS) scores of 0–1, number of bone metastases, rate of vertebral body metastasis, rate of clinical T1–2 disease, frequency of adenocarcinoma, and rate of mutations in the gene encoding the epidermal growth factor receptor (EGFR) (Table 1).
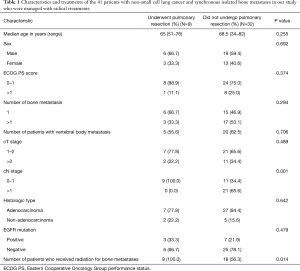
Full table
Details of the nine NSCLC patients in our study who underwent curative-intent surgical resection of their primary tumors are shown in Table 2. They included six men and three women with a median age of 65 years (range, 51–76 years). Eight of the nine patients had ECOG PS scores of 0–1 and all had clinical N0–1 disease. Six patients (66.7%) had a single metastatic lesion in the bone, 1 (11.1%) had two lesions, and 2 (22.2%) had 3 lesions before undergoing primary tumor resection. Two of the nine patients underwent bilobectomies and seven underwent lobectomies. The most prevalent histological type was adenocarcinoma in 7 patients (77.8%); the remaining 2 (22.2%) had squamous cell carcinoma. One patient had clinical N0-pathological N2 lung cancer. Two of the nine patients had been treated with chemoradiotherapy preoperatively, 5 were treated chemoradiotherapy postoperatively (including one preoperative chemoradiotherapy recipient), 3 underwent radiotherapy alone for bone metastases, and 1 did not receive any adjuvant therapy. Both radiotherapy doses for patient number 6 (who received pre- and postoperative chemoradiotherapy) were 45 Gy in 15 fractions (Table 2).
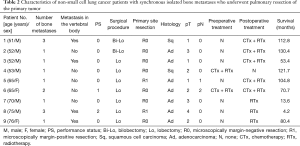
Full table
Survival
The median follow-up time was 19.6 months (range, 1.2–130.4 months) for all patients, 80.4 months (range, 60.4–130.4 months) for surviving patients, and 12.0 months (range, 1.2–71.7 months) for those who had died by the time of analysis. The median OS and five-year OS rate among all 41 patients were 19.6 months (range, 1.2–130.4 months) and 26.8%, respectively. Moreover, the median PFS and five-year PFS rate were 8.2 months (range, 0.6–130.4 months) and 14.6%, respectively.
The durations of treatment after curative-intent surgical resection of the primary lung tumor in our patients are shown in Figure 2. The median OS of the nine patients who underwent curative-intent surgical resection of their primary tumors was 80.4 months (range, 4.2–132.2 months); 5 patients (55.6%) remain alive as of this writing (4 without additional treatment). Furthermore, these 9 patients had five-year OS and PFS rates of 66.7% and 55.6%, respectively (Figure 3). The five-year DFS rate of all nine patients was 44.4%, and the median treatment-free period was 57.1 months (range, 0–123.2 months). Among all 41 NSCLC patients with synchronous isolated bone metastases in our study, all who achieved five-year DFS had undergone resection of the primary pulmonary tumor.
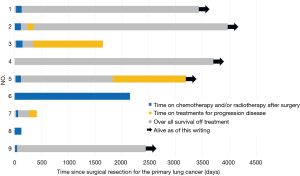
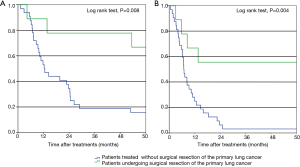
Risk factors associated with survival
Results of the analysis of factors that are predictive of OS are shown in Table 3. On univariate analysis, the favorable prognostic factors were primary lung tumor resection [hazard ratio (HR) =4.51, 95% CI, 1.35–15.1, P=0.014], EGFR mutation (HR =3.60, 95% CI 1.24–10.4, P=0.018), and an ECOG PS score of 0–1 (HR =2.73, 95% CI, 1.20–6.23, P=0.017). On multivariate analysis, the predictors of OS were found to be primary lung tumor resection (HR =4.18, 95% CI, 1.20–14.6, P=0.025) and EGFR mutation (HR =3.30, 95% CI, 1.08–10.1, P=0.036). The factors that were predictive of PFS among all 41 patients are shown in Table 4. Univariate analysis revealed that primary lung tumor resection (HR =4.26, 95% CI, 1.47–12.3, P=0.008) and clinical T1–2 stage lung cancer (HR =2.22, 95% CI, 1.10–4.47, P=0.026) were predictive of PFS; however, only primary lung tumor resection was a significant predictor of five-year PFS on multivariate analysis (HR =3.75, 95% CI, 1.27–11.0, P=0.016).
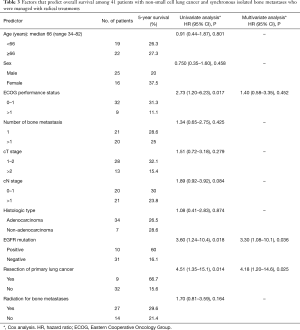
Full table
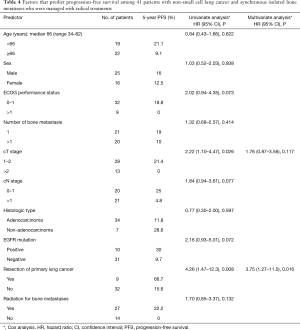
Full table
Discussion
Oligometastasis is defined as one or a limited number of distant metastases that, along with the primary tumor, can potentially be treated with curative strategies including multimodal treatments (4,7). Although the outcomes of patients who underwent curative-intent pulmonary resection for lung cancer with oligometastases have been investigated; such studies focused on synchronous solitary brain or adrenal metastases. In contrast, only a few studies on the outcomes of such patients with isolated bone-metastatic lung cancer have been performed (5-9). The aforementioned study by Endo and colleagues (5) investigated patients with clinical T12N01 lung cancer with a single-organ metastatic lesion in the brain, adrenal gland, lung, or kidney. Yamaguchi and colleagues (7) reported that the five-year PFS and OS rates in patients with synchronous M1b-cStage IV NSCLC who underwent curative-intent pulmonary resection plus treatment of any kind for their metastases were 14.5% and 41.7%, respectively; only three of their patients had bone metastases. Congedo and colleagues (8) reported that the five-year OS of NSCLC patients with oligometastases who underwent curative-intent treatment (79.2% of whom underwent pulmonary resection) was 24%. In our present study, the five-year OS and PFS rates of NSCLC patients with synchronous isolated bone metastases who underwent curative-intent surgical resection of the primary lung tumor were 66.7% and 55.6%, respectively; these patients achieved longer survival than those in previous studies. Furthermore, the five-year DFS of these patients was 44.4%, and the median lung cancer treatment-free duration was 57.1 months (range, 0–123.2 months). As such, NSCLC patients with clinical N0–1 stage who have synchronous isolated bone-metastatic disease are good candidates for curative-intent surgical resection of the primary lung tumor.
Primary lung tumor resection and EGFR mutation were significant predictors of OS in our patients on multivariate analysis; the former was also a significant predictor of PFS. It was previously reported that patients with EGFR mutations have high response rates and long PFS intervals when treated with EGFR TKIs (18). On the other hand, previous studies suggested that local treatment can extend OS in patients with oligometastatic lung cancer more effectively than chemotherapy and/or EGFR-TKIs without local treatment (19,20). In our study, patients who underwent curative-intent surgical resection of their primary lung tumors achieved acceptable five-year OS and PFS rates (66.7% and 55.6%, respectively). On the other hand, three of the 32 patients who did not undergo primary lung tumor resection were treated with chemoradiotherapy for primary lung tumor; all progressed within five years and only one (who had adenocarcinoma) was alive five years after primary treatment. These data suggest that surgical resection of the primary lung tumor is beneficial for NSCLC patients with synchronous isolated bone metastasis, although it is difficult to directly compare the outcomes of patients who underwent curative-intent surgical resection of their primary lung tumors to those who did not undergo this procedure because of our small sample size and differences in patient characteristics.
The complete excision of the primary lung tumor combined with local tumor control of the bone metastases using high-dose radiation significantly extended the survival of our patients. Li and colleagues (21) reported that the initial site of progression was the lung in 66.7% of all their patients with NSCLC who were treated with EGFR-TKIs. In our study, 48% of patients who were treated with chemotherapy and/or radiotherapy without having undergone surgical resection of the primary lung tumor had initial progression in the lung.
De Felice and colleagues (15) reported that radiotherapy for bone metastasis is performed primarily to relieve pain as well as to control bone fractures and spinal cord compression. Their recommended radiotherapy schedules to achieve these aims are 18–36 Gy in 3–6 fractions for painful uncomplicated bone metastases and either 20 Gy in five fractions or 30 Gy in 10 fractions for pathological fractures or spinal cord compression. Zang and colleagues (20) reported that OS tended to improve in patients with stage IV NSCLC and bone metastases who underwent radiotherapy, although they did not specify their dosing regimen. In our study, the median total radiotherapy dose for bone metastases was 40 Gy (range, 25–50 Gy), which was a higher dose than that used in previous studies. Moreover, the median total radiotherapy dose for vertebral body metastases was 39 Gy (range, 25–50 Gy). No patients in our study experienced complications severe enough to require stopping treatment. This indicated that one or a small number of isolated NSCLC bone metastases can be partially controlled via high-dose radiotherapy.
There were several limitations associated with our study. First, it was of a retrospective design; hence, the patients who underwent surgical resection of the primary lung tumor may have been subject to selection bias. However, the criteria for selecting such patients are not well defined; in our study, these criteria were an ECOG PS score of 0–1, having one or a small number of isolated bone metastases, clinical N0–1 stage, and an expectation of successful complete resection. Second, the present study was of a relatively small population; for this reason, our cohort may not be adequately representative of broader pools of patients with synchronous isolated bone-metastatic NSCLC. Additional studies of patients with synchronous isolated bone-metastatic NSCLC who undergo resection of the primary lung tumor will be necessary to acquire robust evidence to confirm the benefit of this radical intervention.
In conclusion, both surgical resection of the primary tumor and EGFR mutation positivity predict longer survival times in NSCLC patients with synchronous isolated bone metastases. As such, clinical N0–1 NSCLC patients with such metastases may be good candidates for resection of the primary pulmonary tumor.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study was approved by the National Hospital Organization, Hokkaido Cancer Center Institutional Review Board (approval number: 302-167); the requirement to obtain informed consent was waived.
References
- Azzoli CG, Giaccone G, Temin S. American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non-Small-Cell Lung Cancer. J Oncol Pract 2010;6:39-43. [Crossref] [PubMed]
- Sculier JP. Nonsmall cell lung cancer. Eur Respir Rev 2013;22:33-6. [Crossref] [PubMed]
- De Ruysscher D, Wanders R, van Baardwijk A, et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: long-term results of a prospective phase II trial (Nct01282450). J Thorac Oncol 2012;7:1547-55. [Crossref] [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Endo C, Hasumi T, Matsumura Y, et al. A prospective study of surgical procedures for patients with oligometastatic non-small cell lung cancer. Ann Thorac Surg 2014;98:258-64. [Crossref] [PubMed]
- Loi M, Mazzella A, Mansuet-Lupo A, et al. Synchronous Oligometastatic Lung Cancer Deserves a Dedicated Management. Ann Thorac Surg 2019;107:1053-9. [Crossref] [PubMed]
- Yamaguchi M, Edagawa M, Suzuki Y, et al. Pulmonary Resection for Synchronous M1b-cStage IV Non-Small Cell Lung Cancer Patients. Ann Thorac Surg 2017;103:1594-9. [Crossref] [PubMed]
- Congedo MT, Cesario A, Lococo F, et al. Surgery for oligometastatic non-small cell lung cancer: long-term results from a single center experience. J Thorac Cardiovasc Surg 2012;144:444-52. [Crossref] [PubMed]
- Xu Q, Wang Y, Liu H, et al. Treatment outcome for patients with primary NSCLC and synchronous solitary metastasis. Clin Transl Oncol 2013;15:802-9. [Crossref] [PubMed]
- Deberne M, Ropert S, Billemont B, et al. Inaugural bone metastases in non-small cell lung cancer: a specific prognostic entity? BMC Cancer 2014;14:416. [Crossref] [PubMed]
- Zhang L, Gong Z. Clinical characteristics and prognostic factors in bone metastases from lung cancer. Med Sci Monit 2017;23:4087-94. [Crossref] [PubMed]
- Bates JE, Milano MT. Prognostic significance of sites of extrathoracic metastasis in patients with non-small cell lung cancer. J Thorac Dis 2017;9:1903-10. [Crossref] [PubMed]
- Patrini D, Panagiotopoulos N, Bedetti B, et al. Surgical approach in oligometastatic non-small cell lung cancer. Ann Transl Med 2018;6:93. [Crossref] [PubMed]
- Tönnies M, Pfannschmidt J, Bauer TT, et al. Metastasectomy for synchronous solitary non-small cell lung cancer metastases. Ann Thorac Surg 2014;98:249-56. [Crossref] [PubMed]
- De Felice F, Piccioli A, Musio D, et al. The role of radiation therapy in bone metastases management. Oncotarget 2017;8:25691-9. [Crossref] [PubMed]
- Travis WD, Brambilia E, Konrad Müller-Hermelink HK, et al. editors. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC, 2004.
- Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours. New York: Wiley-Blackwell, 2009:136-47.
- Mayekar MK, Bivona TG. Current landscape of targeted therapy in lung cancer. Clin Pharmacol Ther 2017;102:757-64. [Crossref] [PubMed]
- Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 2012;7:1807-14. [Crossref] [PubMed]
- Zhang R, Li P, Li Q, et al. Radiotherapy improves the survival of patients with stage IV NSCLC: a propensity score matched analysis of the SEER database. Cancer Medicine 2018;7:5015-26. [Crossref] [PubMed]
- Li X, Qi H, Qing G, et al. Microwave ablation with continued EGFR tyrosine kinase inhibitor therapy prolongs disease control in non-small-cell lung cancers with acquired resistance to EGFR tyrosine kinase inhibitors. Thorac Cancer 2018;9:1012-7. [Crossref] [PubMed]



