
Lung cancer screening: tell me more about post-test risk
Lung cancer screening (LCS) by low-dose computed tomography (LDCT) can save lives because LC is detected in its early asymptomatic stage (1). We have come a long way since the first results of the national lung screening trial (NLST) (2), now confirmed in Europe by volumetric LDCT against control arm by the Nederlands Leuvens Longkanker Screenings Onderzoek (NELSON) (3) and the multicentric Italian lung detection (MILD) (4). The literature shows a substantial variability of LC risk even among screenees selected with strict risk criteria (5-7). Furthermore, the evolving research in LCS provides reinforced evidence on prolonged LCS for continuous and incremental reduction of lung cancer mortality (4,8,9). In such scenario, the relentless development and optimization of LCS practice is about reduction of false positives and radiation exposure, while refining efficiency (10-15).
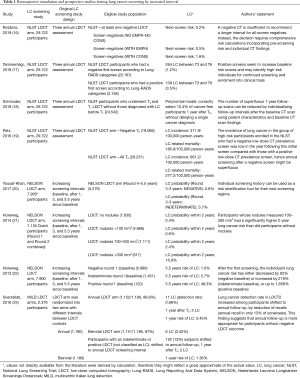
LCS with longer-than-one-year interval between LDCT rounds looks like an interesting option for such purpose. Longer LCS interval is also known as low intensity approach. Here comes the interesting analysis of Robbins et al. who retrospectively interrogated the NLST database with the aim of defining post-test risk of lung cancer by description of LDCT findings (16) (Table 1).
Full table
Robbins et al. classified each screenee according to negative LDCT outcome (NLST criteria: solid nodule <4 mm) and radiological signs of subjective susceptibility to lung damage: emphysema and consolidation. Any LDCT was included in the analysis of post-test risk stratification, either baseline (1 for each screenee) or incidence round (up to 2 for each screenee). As such, the selected descriptors of post-test risk were applied on the top of a validated pre-test risk model, the lung cancer risk assessment tool (LCRAT) (24). The LCRAT is a comprehensive pre-test risk model developed in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial and the NLST population, as well as data from the National Health Interview Survey (NHIS). Pre-test risk factors for the development of this model included several covariates: age, gender, ethnicity, education, body mass index, smoking history (pack-years, status, years since quitting, years of smoking, and cigarettes per day), self-reported emphysema, and family history of LC. The LCRAT was selected among the best performers in a model study including nine of the most authoritative pre-test risk models. It was also externally validated with expected–observed ratio 0.97 (in Non-Hispanic white) and area under the curve above 0.75 when applying screening eligibility threshold of 2% LC risk over 5 years (25). In the population selected by Robbins, the next-screen risk of LC by LCRAT was 0.3%. The merging of LCRAT with post-test variables was named LCRAT + CT, intriguingly this composite system returned heterogeneous LC risk stratification among LDCT negative subjects. The post-test implementation could outline three major categories of LC risk among screenees with negative LDCT outcome: 1.6% risk in the 0.6% of screenees with consolidation, 0.5% risk in the 30% of screenees with emphysema, and as low as 0.2% risk in the 70% of screenees with neither. Thereof, both consolidation and emphysema seem to confer higher-than-expected risk of LC in 1 year, allegedly as a consequence of subjective susceptibility to tissue damage. Conversely, screenees with negative LDCT and neither emphysema or consolidation showed a lower-than-expected risk of LC in one year. The LCRAT+CT could predict LC at next screen, whilst it was not accurate for interval cancer. By assuming a 0.3% risk threshold for preference sensitive LCS (26), Robbins et al. found that LCRAT + CT assigned 58% of screenees below such threshold. Noteworthy, this selection yielded potential reduction of false positives by 50% at the cost of about 24% delayed diagnosis of LC in case of low intensity approach. Intuitively, further reduction of the risk threshold would have reduced the proportion of delayed diagnosis, however also a lower optimization of false positive would have followed, too.
The analysis from Robbins et al. comes as a refinement of a previous study by Patz et al. who retrospectively stratified LC risk according to lone LDCT outcome by NLST criteria (nodule ≥4 mm) (19). Already, Patz et al. could describe a substantial drop of LC risk in subjects with negative baseline screen, selecting about 70% of screenees with 0.34% risk of LC in 1 year and, therefore, addressable to low intensity approach by biennial rounds. It should be underscored that LCRAT could stratify a pre-test risk of 0.3% in negative screenees (this was the expected risk in this selection based on socio-demographic covariates), which was substantially consistent by the post-test risk 0.34% reported by Patz (this was the risk based on the LDCT outcome, without any risk scaling by socio-demographic data). This figure comes as a further confirmation of reliability of LCRAT for selection of screenees. Then, if post-test radiological descriptors (different from nodule, namely emphysema or consolidation) are applied, the composite pre-test and post-test system LCRAT+CT provides even more accurate apportioning of the next-screen risk of LC in 1 year. The integration by Robbins of these two different perspectives on the NLST population suggests that radiological descriptors could master LCS approach by low intensity, either they represent a nodule (potentially a cluster of neoplastic cells) or emphysema and consolidation (representing some increased susceptibility of pulmonary tissue damage). Such evidence unveiled by Robbins et al. comes as substantial potential game-changer in the path towards personalized medicine/prevention in the purpose of the most efficient approach to population LCS.
This retrospective exercise by LCRAT + CT offers a valuable opportunity for comparison with previous data. Unlike Robbins, Tammemägi et al. showed that a continuously negative LDCT result through the three NLST time-points was still associated with >1.5% risk in subjects that featured a pre-test risk >2.6% by the model called PLCOm2012 (17). The major difference between the two analysis is found in the pre-test risk: very low for Robbins et al. and quite high for Tammemägi et al. Therefore, the manuscript from Robbins underscores the actual strength of post-test risk stratification, which should be trusted exclusively amongst screenees with relatively low pre-test risk.
Further authors analysed the NLST population with the objective of investigating the feasibility of low intensity LCS. Notably, Schreuder et al. proposed and validated a so called polynomial model, it was derived from the whole NLST dataset, including screenees with either negative or positive LDCT outcome (for this reason these results cannot be raw-paralleled to the analysis of Robbins) (18). This approach showed interesting evidence about the potential reduction of LDCT and the relevant cost in terms of delayed diagnosis. For instance, the polynomial model retrospectively estimated 68% reduction of LDCT at the cost of 25% delayed diagnosis, whilst zero delayed diagnosis could offer a limited 10% potential reduction of LDCT burden. Such figure tightly overlapped the data from Robbins, further confirming that the LCRAT + CT approach is useful in the large proportion of screenees with negative LDCT, at any time during the personal screening history. Of note, the stratification of the whole population by the polynomial model was granted by further post-test variables than the LCRAT + CT (e.g., presence of subsolid nodule, upper lobe location of the nodule, spiculation, and nodule count), which make the polynomial approach more similar to the Brock model published by McWilliams et al. in 2013 (27).
Also, the Brock model was applied to the NLST database for external validation. It resulted in 0.905 area under the curve, but with limited discrimination between benign and malignant cases as analysed by concordance statistic (c-statistic 0.905) (28). Noteworthy, the Brock model appeared to overestimate the probability of cancer in the NLST population. Following this observation, Winter et al. could recalibrate the Brock model in the specific NLST population and found significantly improved performance for prediction of malignancy (highest c-statistic 0.914). While the LCRAT + CT addressed subjects with negative LDCT, the polynomial model and NLS-adapted Brock model have a complementary use, namely addressing the risk in all subjects with particular focus on those with discrete nodules. A side by side comparison of these models would probably show the ideal way to their harmonized contribution for refined post-test risk stratification. Still, their application outside of the NLST dataset would grant further validation and, eventually, recalibration.
Beyond retrospective simulations in the NLST population, prospective trials with low intensity are found in the literature. The low intensity approach was explicitly addressed by the MILD trial, where annual and biennial algorithms were compared (29). Biennial screening in subjects with negative LDCT prospectively granted 86% reduction of first-year LDCT repeats and 38% of all repeats in the biennial arm. Noteworthy, this approach allowed 32% reduction of the total LDCT burden in the biennial arm compared with annual arm, and 37% reduction among subjects with negative baseline LDCT.
The NELSON trial included subsequently lower intensity: 1-, 2-, and 2.5-year intervals. Despite the absence of a control arm with annual screening and the ageing of the population through the progressively lower intensity, also this trial showed that biennial screening is approachable in subjects with negative LDCT outcome (30). Nonetheless, a substantial increase in interval cancers was witnessed in the last six months of the 2.5-year interval screen, hence this extremely low-intensity approach was deemed hazardous.
The most extreme approach to low intensity screening is currently underway at the bioMILD trial, where over 4,000 screenees underwent baseline LDCT and blood sampling: triennial round was assigned in case of negative LDCT and negative blood test (31). Noteworthy, the bioMILD algorithm encompasses biological risk stratification by circulating miRNA (32), which allowed increase of volumetric threshold for definition of negative LDCT and prolonged interval for follow up of indeterminate findings (e.g., 1 year).
Great interest is directed toward optimization of LCS also by reduction of overall radiation burden, here administered to healthy individuals “the screenees”. The risk of radiation-induced cancer has been considered not negligible, yet acceptable (33). Nonetheless, obtaining diagnostic images at the lowest radiation exposure is ethical. Recent advances in CT scanners technology allowed to further optimize CT scanning protocols, reaching sub-millisievert levels (34). Several researches tested the impact of the tin-filter technique for dramatic reduction of radiation burden in LCS, both by anthropomorphic phantom experiments (35) and by human patients (36). The use of the tin-filter technique is everything but obvious, especially for application of semi-quantitative software and for the detection of the full range of nodule density. Future studies are fostered testing the possible implementation of ultra-low dose CT in LCS, in particular for investigation of nodule detection as well as characterization of parenchymal abnormalities (i.e., emphysema).
The current technological development in radiology finds a hotspot in LCS (37), where automatic nodule detection and volumetry were developed and applied to help accuracy of the LDCT test (38-43). Volumetry of nodule appears to mismatch diameter (44), whilst volume is deemed a standard of reference (45,46). In 2019, the Lung Reporting And Data System (LungRADS) released the 1.1 version, where a major novelty was represented by inclusion of volumetric thresholds (47). One intrinsic limit of the analysis from Robbins et al. comes with the linear (non-volumetric) manual measurement of nodule provided by the NLST database. Any study based on NLST data is obliged to this methodological setting (18,19). Otherwise European studies provide a wealth of information about post-test risk stratification by volumetric LDCT, assisted detection of nodule, and semi-automatic volumetry of nodule (48,49). The volumetric approach is becoming more and more endorsed worldwide, risk models for future application in LCS will have to cope with this method.
Beside volumetry, further nodule-derived post-test metrics potentially could contribute in composite risk models, for instance radiomics. Radiomics is a radiology research field focused on detecting associations between quantitative descriptors from images and clinical parameters (50). A number of studies reported improved diagnostic accuracy by radiomics, notably for discrimination between pulmonary cancer and benign nodules (51). In a NLST-based study, a computer aided diagnosis algorithm was capable of increasing positive predictive values for small pulmonary nodules (52). This is a brand burgeoning frontier of radiology, the reliability of which is still to be confirmed.
A further scientific question arises from the present paper of Robbins: was self-reported emphysema of LCRAT consistent with emphysema detected at CT? This is one of several akin questions that might apply to the context of LCS: radiological verification of self-disclosed variables. One issue from this area of investigation has already been outlined on the MILD data: self-disclosed asbestos exposure was compared with pleural plaques at LDCT (53). The vast majority of screenees with pleural plaques did not report self-disclosed exposure to asbestos and pleural plaques were associated with higher risk of lung cancer mortality. Is radiological post-test risk stratification again a reference for confirmation of pre-test variables? Should radiologist provide an oversight on the reliability of self-disclosed information?
In conclusion, the way to optimal LCS practice is oriented towards relevant apportioning of LC risk by comprehensive risk models. The analysis by Robbins et al. outlines personalized risk profile by relatively simple approach, the so called LCRAT + CT, which confirms the feasibility of low intensity screening in a high-risk population such as NLST. Let us be prepared for optimization of long-term lung cancer screening by LDCT with personalized intensity.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Schabath MB, Aberle DR. MILD trial, strong confirmation of lung cancer screening efficacy. Nat Rev Clin Oncol 2019;16:529-30. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- De Koning H, van der Aalst C, Ten Haaf K, et al. Effects of Volume CT Lung Cancer Screening: Mortality Results of the NELSON Randomised-Controlled Population Based Trial. J Thorac Oncol 2018;13:S185. [Crossref]
- Pastorino U, Silva M, Sestini S, et al. Prolonged Lung Cancer Screening Reduced 10-year Mortality in the MILD Trial. Ann Oncol 2019. [Epub ahead of print]. [Crossref]
- Kovalchik SA, Tammemagi M, Berg CD, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med 2013;369:245-54. [Crossref] [PubMed]
- Wille MM, Dirksen A, Ashraf H, et al. Results of the Randomized Danish Lung Cancer Screening Trial with Focus on High-Risk Profiling. Am J Respir Crit Care Med 2016;193:542-51. [Crossref] [PubMed]
- Becker N, Motsch E, Trotter A, et al. Lung cancer mortality reduction by LDCT screening-Results from the randomized German LUSI trial. Int J Cancer 2019. [Epub ahead of print]. [Crossref] [PubMed]
- National Lung Screening Trial Research Team. Lung Cancer Incidence and Mortality with Extended Follow-up in the National Lung Screening Trial. J Thorac Oncol 2019. [Epub ahead of print]. [PubMed]
- Rota M, Pizzato M, La Vecchia C, et al. Efficacy of lung cancer screening appears to increase with prolonged intervention: results from the MILD trial and a meta-analysis. Ann Oncol 2019;30:1040-3. [Crossref] [PubMed]
- Henschke CI, Yip R, Yankelevitz DF, et al. Definition of a positive test result in computed tomography screening for lung cancer: a cohort study. Ann Intern Med 2013;158:246-52. [Crossref] [PubMed]
- CT Volumetry Biomarker Ctte. Available online: http://qibawiki.rsna.org/index.php/CT_Volumetry_Biomarker_Ctte
- European Society of Thoracic Imaging (ESTI). Chest CT for Lung Cancer Screening. 2019. Accessed July 20 2019. Available online: https://www.myesti.org/content-esti/uploads/ESTI-LCS-technical-standards_2019-06-14.pdf
- Aberle DR. Implementing lung cancer screening: the US experience. Clin Radiol 2017;72:401-6. [Crossref] [PubMed]
- Tomonaga Y, Ten Haaf K, Frauenfelder T, et al. Cost-effectiveness of low-dose CT screening for lung cancer in a European country with high prevalence of smoking-A modelling study. Lung Cancer 2018;121:61-9. [Crossref] [PubMed]
- Kauczor HU, Baird AM, Blum T, et al. ESR-ERS joint statement paper on Lung Cancer Screening. Eur Respir J 2019. in press.
- Robbins HA, Berg CD, Cheung LC, et al. Identification of candidates for longer lung cancer screening intervals following a negative low-dose computed tomography result. J Natl Cancer Inst 2019;111:996-9. [Crossref] [PubMed]
- Tammemägi MC, Ten Haaf K, Toumazis I, et al. Development and Validation of a Multivariable Lung Cancer Risk Prediction Model That Includes Low-Dose Computed Tomography Screening Results: A Secondary Analysis of Data From the National Lung Screening Trial. JAMA Netw Open 2019;2:e190204. [Crossref] [PubMed]
- Schreuder A, Schaefer-Prokop CM, Scholten ET, et al. Lung cancer risk to personalise annual and biennial follow-up computed tomography screening. Thorax 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Patz EF Jr, Greco E, Gatsonis C, et al. Lung cancer incidence and mortality in National Lung Screening Trial participants who underwent low-dose CT prevalence screening: a retrospective cohort analysis of a randomised, multicentre, diagnostic screening trial. Lancet Oncol 2016;17:590-9. [Crossref] [PubMed]
- Yousaf-Khan U, van der Aalst C, de Jong PA, et al. Risk stratification based on screening history: the NELSON lung cancer screening study. Thorax 2017;72:819-24. [Crossref] [PubMed]
- Horeweg N, Scholten ET, de Jong PA, et al. Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncol 2014;15:1342-50. [Crossref] [PubMed]
- Horeweg N, van der Aalst CM, Vliegenthart R, et al. Volumetric computed tomography screening for lung cancer: three rounds of the NELSON trial. Eur Respir J 2013;42:1659-67. [Crossref] [PubMed]
- Sverzellati N, Silva M, Calareso G, et al. Low-dose computed tomography for lung cancer screening: comparison of performance between annual and biennial screen. Eur Radiol 2016;26:3821-9. [Crossref] [PubMed]
- Katki HA, Kovalchik SA, Berg CD, et al. Development and Validation of Risk Models to Select Ever-Smokers for CT Lung Cancer Screening. JAMA 2016;315:2300-11. [Crossref] [PubMed]
- Katki HA, Kovalchik SA, Petito LC, et al. Implications of Nine Risk Prediction Models for Selecting Ever-Smokers for Computed Tomography Lung Cancer Screening. Ann Intern Med 2018;169:10-9. [Crossref] [PubMed]
- Caverly TJ, Cao P, Hayward RA, et al. Identifying Patients for Whom Lung Cancer Screening Is Preference-Sensitive: A Microsimulation Study. Ann Intern Med 2018;169:1-9. [Crossref] [PubMed]
- McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013;369:910-9. [Crossref] [PubMed]
- Winter A, Aberle DR, Hsu W. External validation and recalibration of the Brock model to predict probability of cancer in pulmonary nodules using NLST data. Thorax 2019;74:551-63. [Crossref] [PubMed]
- Pastorino U, Sverzellati N, Sestini S, et al. Ten-year results of the Multicentric Italian Lung Detection trial demonstrate the safety and efficacy of biennial lung cancer screening. Eur J Cancer 2019;118:142-8. [Crossref] [PubMed]
- Yousaf-Khan U, van der Aalst C, de Jong PA, et al. Final screening round of the NELSON lung cancer screening trial: the effect of a 2.5-year screening interval. Thorax 2017;72:48-56. [Crossref] [PubMed]
- Plasma microRNA Profiling as First Line Screening Test for Lung Cancer Detection: a Prospective Study (bioMILD). Available online: https://clinicaltrials.gov/ct2/show/study/NCT02247453
- Sozzi G, Boeri M, Rossi M, et al. Clinical utility of a plasma-based miRNA signature classifier within computed tomography lung cancer screening: a correlative MILD trial study. J Clin Oncol 2014;32:768-73. [Crossref] [PubMed]
- Rampinelli C, De Marco P, Origgi D, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ 2017;356:j347. [Crossref] [PubMed]
- Martini K, Barth BK, Nguyen-Kim TD, et al. Evaluation of pulmonary nodules and infection on chest CT with radiation dose equivalent to chest radiography: Prospective intra-individual comparison study to standard dose CT. Eur J Radiol 2016;85:360-5. [Crossref] [PubMed]
- Milanese G, Silva M, Frauenfelder T, et al. Comparison of ultra-low dose chest CT scanning protocols for the detection of pulmonary nodules: a phantom study. Tumori 2019;105:394-403. [Crossref] [PubMed]
- Takahashi EA, Koo CW, White DB, et al. Prospective Pilot Evaluation of Radiologists and Computer-aided Pulmonary Nodule Detection on Ultra-low-Dose CT With Tin Filtration. J Thorac Imaging 2018;33:396-401. [PubMed]
- Silva M, Milanese G, Seletti V, et al. Pulmonary quantitative CT imaging in focal and diffuse disease: current research and clinical applications. Br J Radiol 2018;91:20170644. [Crossref] [PubMed]
- Nair A, Baldwin DR, Field JK, et al. Measurement methods and algorithms for the management of solid nodules. J Thorac Imaging 2012;27:230-9. [Crossref] [PubMed]
- Ritchie AJ, Sanghera C, Jacobs C, et al. Computer Vision Tool and Technician as First Reader of Lung Cancer Screening CT Scans. J Thorac Oncol 2016;11:709-17. [Crossref] [PubMed]
- Silva M, Schaefer-Prokop CM, Jacobs C, et al. Detection of Subsolid Nodules in Lung Cancer Screening: Complementary Sensitivity of Visual Reading and Computer-Aided Diagnosis. Invest Radiol 2018;53:441-9. [Crossref] [PubMed]
- Devaraj A, van Ginneken B, Nair A, et al. Use of Volumetry for Lung Nodule Management: Theory and Practice. Radiology 2017;284:630-44. [Crossref] [PubMed]
- Nair A, Screaton NJ, Holemans JA, et al. The impact of trained radiographers as concurrent readers on performance and reading time of experienced radiologists in the UK Lung Cancer Screening (UKLS) trial. Eur Radiol 2018;28:226-34. [Crossref] [PubMed]
- Milanese G, Eberhard M, Martini K, et al. Vessel suppressed chest Computed Tomography for semi-automated volumetric measurements of solid pulmonary nodules. Eur J Radiol 2018;101:97-102. [Crossref] [PubMed]
- Heuvelmans MA, Walter JE, Vliegenthart R, et al. Disagreement of diameter and volume measurements for pulmonary nodule size estimation in CT lung cancer screening. Thorax 2018;73:779-81. [Crossref] [PubMed]
- Oudkerk M, Devaraj A, Vliegenthart R, et al. European position statement on lung cancer screening. Lancet Oncol 2017;18:e754-66. [Crossref] [PubMed]
- Callister ME, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015;70 Suppl 2:ii1-54. [Crossref] [PubMed]
- ACoR. Lung-Screening Reporting and Data System (LungRADS) Version 1.1. 2019. Accessed July 5 2019. Available online: https://www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/LungRADSAssessmentCategoriesv1-1.pdf?la=en
- Silva M, Pastorino U, Sverzellati N. Lung cancer screening with low-dose CT in Europe: strength and weakness of diverse independent screening trials. Clin Radiol 2017;72:389-400. [Crossref] [PubMed]
- Horeweg N, van Rosmalen J, Heuvelmans MA, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol 2014;15:1332-41. [Crossref] [PubMed]
- Rizzo S, Botta F, Raimondi S, et al. Radiomics: the facts and the challenges of image analysis. Eur Radiol Exp 2018;2:36. [Crossref] [PubMed]
- Wilson R, Devaraj A. Radiomics of pulmonary nodules and lung cancer. Transl Lung Cancer Res 2017;6:86-91. [Crossref] [PubMed]
- Huang P, Park S, Yan R, et al. Added Value of Computer-aided CT Image Features for Early Lung Cancer Diagnosis with Small Pulmonary Nodules: A Matched Case-Control Study. Radiology 2018;286:286-95. [Crossref] [PubMed]
- Silva M, Sverzellati N, Colombi D, et al. Pleural plaques in lung cancer screening by low-dose computed tomography: prevalence, association with lung cancer and mortality. BMC Pulm Med 2017;17:155. [Crossref] [PubMed]


