
Clinical predictors of asthmatics in identifying subgroup requiring long-term tiotropium add-on therapy: a real-world study
Introduction
Tiotropium is a long-acting muscarinic antagonist (LAMA) for managing chronic obstructive pulmonary disease (COPD), improving patient’s quality of life as well as lung function, and controlling disease exacerbation (1,2). It also acts as an effective long-acting bronchodilator in the stepwise asthmatic management, and thus has been included in Global Initiative for Asthma (GINA) guidelines since 2015. Tiotropium has shown favorable effects on asthma control in a few randomized control trials and systemic reviews (3-5). The PrimoTinA-asthma study [two replicate randomized controlled trials (RCTs) involving 912 patients with poorly-controlled asthma, who were receiving inhaled glucocorticoids and long-acting beta2-agonists (LABAs)] indicated that the addition of tiotropium significantly extended the time to first severe exacerbation, and improved peak and trough forced expiratory volume-one second (FEV1) at week 24 (3). The MezzoTina-asthma trials [two RCTs involving 2,013 patients with moderate and symptomatic asthma, and under moderate inhaled corticosteroids (ICS)] showed that tiotropium or salmeterol add-on therapy had comparable efficacy on peak or trough FEV1 and Asthma Control Questionnaire (ACQ-7) response rate (6). The GraziaTinA-asthma trial (included 464 patients with mild to moderate asthma under low to medium ICS) indicated that tiotropium add-on therapy significantly increased peak FEV1 and peak expiratory flow (PEF) (7). Based on these studies, tiotropium played a role across varying asthma severities in poor-controlled asthmatics (who at least received ICS or ICS/LABA) in terms of delaying asthma exacerbation and improving pulmonary function.
However, asthma is characterized by heterogeneous clinical phenotypes (e.g., airway inflammation as Th-2 high and Th-2 low phenotypes). Despite clinical phenotypes have made progress in understanding the variety of asthma pathophysiologies, the majority asthmatics are still treated with “one size fits all” approach according to the GINA guidelines. As no large RCTs are available so far, clinical phenotype subgroups analyses may offer a better knowledge about beneficial phenotype-specific tiotropium add-on therapies. A subgroup analysis (performed by Kerstjens et al.) based on two phase III PrimoTinA-asthma studies indicated that tiotropium add-on therapy was effective for asthma management regardless of age, sex, body mass index (BMI), FEV1, serum IgE levels and eosinophil counts (8). Casale et al. also reported that according to the subgroup analysis on 4 large RCTs, the efficacy of tiotropium add-on therapy is independent of Th-2 phenotype (9).
In addition, Peters et al. reported that predictors of positive clinical response to tiotropium included positive airway obstruction and albuterol response (10), while Liccardi et al. indicated that tiotropium was more effective in asthmatics characterized by increased cholinergic activity (11). Moreover, a small study involving 4 weeks of add-on tiotropium for severe asthmatics under high-dose ICSs demonstrated that the percentage of neutrophils in sputum were positively correlated with the improvement of lung functions (12). Our previous study involving tiotropium add-on therapy for uncontrolled asthmatics under medium-to-high ICS plus LABA also showed that a 3-month tiotropium add-on therapy led to clinical characteristics-independent asthma control (except for IgE >430 IU/mL, which was a negative predictor) (13). In this time, we have just analyzed factors contributing to need for long-term use of tiotropium added on ICS and ICS/LABA in asthmatic patients using the same cohort. Although GINA guidelines suggest that a step-down treatment for asthmatics is recommended once control is achieved, some asthmatics (e.g., those with Th-2 low phenotype) may not step down or withdraw from tiotropium easily due to different clinical phenotypes. The goal of this study aims to identify predictors (in response to tiotropium add-on therapy) based on asthma clinical phenotypes.
Methods
Study design
The is a retrospective study design involving all patients visited the Division of Pulmonary and Critical Care Medicine, China Medical University Hospital, Taiwan between July 2016 and July 2018. The study was approved by the China Medical University Hospital Institutional Review Board (CMUH103-REC1-112), and written consent was obtained from all patients.
Study cohort and data collection
Patients were eligible for this study only if all of the following criteria have been met: (I) aged eighteen years old or above; (II) diagnosed of asthma not only based on the clinical symptoms and signs, but to seek evidence of variable airflow obstruction on spirometry (increase in FEV1 >12% and 200 mL after 400 µg of salbutamol administration); (III) uncontrolled asthmatics under maintenance treatment using low- or medium-to-high ICS with LABA for 4 weeks or longer (GINA step 3 or 4), indicating the need of step-up treatment; (IV) tiotropium bromide add-on therapy at a recommended dose of 5 µg once daily via the Respimat Soft MoistTM inhaler according to GINA Guidelines 2018. All the above asthmatics participated in a pay-for-performance (P4P) (also known as “value-based purchasing”) scheme to save healthcare costs and improve their health outcomes. Active communication between the patients, physicians and nurses; regular asthma control tests (ACT); and diet plus exercise-controlled programs were also included in the P4P scheme for asthma care.
Therapeutic options in asthma control were following the recommendations of GINA guidelines. According to the guidelines, for those whose asthma control has not been achieved with low-dose ICS/LABA combination (GINA step 3), tiotropium add-on therapy is the other controller option (step 3 to 4); and for those whose asthma control has not been achieved with medium-to-high ICS/LABA combination (GINA step 4), tiotropium add-on therapy is a preferred controller option (step 4 to 5). Therefore, by analyzing the supplement of controller option among variable clinical phenotype of asthmatics, those who required long-term tiotropium add-on therapy may be identified.
All of the potential clinical predictors were collected, including age, sex, smoking history (yes or no), asthma-and-chronic COPD-overlap (ACO), BMI, the steps of asthma control, pulmonary function test (PFT), ACT score, eosinophil counts, and serum IgE level. ACO is characterized by persistent airflow limitation with several features usually associated with asthma and COPD based on GINA 2018.
Assessments
The PFT and ACT scores [developed since 2004 (14)] were used as asthma control indicators after tiotropium add-on therapy. Patients were required to return to our clinics every month, and ACT assessment was performed 3 months after tiotropium add-on therapy. Subjects were then divided into two subgroups by the improvement in ACT score: one was tiotropium responder (TR), and the other was tiotropium non-responder (TNR). A clinically significant “minimally important difference” (MID) of ACT is a difference equals to or is greater than 3 ACT points between 2 or more subgroups (15). Based on this MID of ACT, TR was defined as asthmatics with ACT score increased more than 3 points after 3-month add-on therapy, while TNR referred to asthmatics with ACT score increased less than 2 points or even ACT score decreased after 3-month add-on therapy.
In TNR group, all of patients stop to use tiotropium 3 months after the initiation of add-on therapy. Those whose asthma was not under control with low-dose ICS plus LABA (GINA step 3) stepped up to medium-to-high-dose ICS plus LABA, and the others whose asthma was not under control with medium-to-high-dose ICS plus LABA (GINA step 4) stepped up to systemic corticosteroid or other alternative therapy. However, these asthma control medications were adjusted at patient’s monthly follow-up visit according to the GINA score. Patients in TR group remained in the tiotropium treatment after 3-month tiotropium add-on therapy, and they were further divided into two subgroups after one-year follow-up: one was ≥1-year tiotropium add-on therapy as the asthma control couldn’t be achieved after the step-down treatment, and the other was <1-year tiotropium add-on therapy due to a successful step-down treatment. Based on the clinical phenotypes (responsiveness to tiotropium), it is supposed to be some clinical characteristics contributing to such difference between the two subgroups.
Statistical analysis
The data were analyzed using SPSS (version 17.0). Continuous variables were reported as mean ± SD and were compared using two-tailed Student’s t-tests or Mann-Whitney U tests. Categorical variables were reported as number of patients and percentages. Differences between categorical variables were evaluated using Chi-square or Fisher’s exact test. Univariate analysis was performed to determine factors that were associated with asthma patient who needed long-term tiotropium to maintain asthma control. Variables then entered into a final model for a stepwise multiple logistic regression analysis. All statistical tests were two-sided, and a P value <0.05 was considered statistically significant.
Results
Patient characteristics
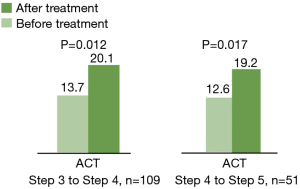
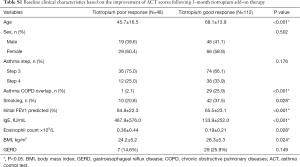
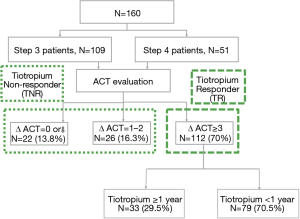
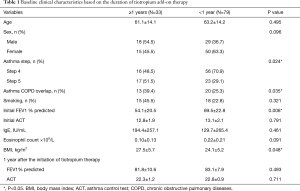
From July 2016 to July 2018, a total of 160 uncontrolled asthma patients lasting for 4 weeks or longer (despite under regular maintenance therapy) were enrolled in the study. Among them, 109 patients were prescribed with low-dose ICS plus LABA (GINA step 3), while 51 patients were prescribed with medium-to-high-dose ICS plus LABA (GINA step 4). After a 3-month tiotropium add-on therapy, the ACT score increased from 13.7 to 20.1 points in the former group (GINA step 3) (P=0.012) and from 12.6 to 19.2 points in the latter group (GINA step 4) (P=0.017) (Figure 1). These subjects were then divided into two subgroups according to the changes in ACT score. Baseline characteristics of these total patients (n=160) were shown in Table S1. One hundred and twelve patients (70%) had good response to tiotropium (TR group) by showing increased ACT score ≥3 points; 48 patients (30%) had poor response to tiotropium (TNR group) with ACT score increased <3 points. In TNR group, 26 patients (16.3%) increased ACT score by 1 to 2 points, while 22 patients (13.8%) displayed unchanged or decreased ACT score. In TR group, a total of 33 patients (29.5%) were treated with tiotropium add-on therapy more than one year after the first 3-month add-on therapy due to the failure of step-down tiotropium therapy; but a total 79 patients (70.5%) successfully stepped down or withdrew from tiotropium add-on therapy (Figure 2). Based on the clinical phenotypes, TR group were further divided into 2 subgroups depending on the duration of tiotropium add-on therapy (i.e., ≥1 or <1 year). Table 1 described baseline characteristics between 2 TR subgroups. Among these 2 TR subgroups, the PFT (FEV1% predicted) were improved after or within one-year tiotropium add-on therapy (54.1%±20.5% to 81.8%±10.6% and 69.5%±22.8% to 83.1%±7.9%); the ACT were also improved after or within one year tiotropium add-on therapy (12.8±1.9 to 22.3±1.2 and 13.1±2.1 to 22.8±0.9). Indicators such as asthma treatment step, ACO, initial FEV1% predicted, and BMI were found significantly different between the two subgroups. Patients required ≥1-year tiotropium add-on therapy not only had a higher proportion of ACO patients (13/33, 39.4%) than <1-year group (20/79, 25.3%) (P=0.035), but had higher BMI than that in <1-year group (27.5±5.7 vs. 24.1±5.2; P=0.048). Moreover, the initial FEV1% predicted in ≥1-year group was significantly lower than the <1-year group (54.1%±20.5% vs. 69.5%±22.8%; P=0.006). Besides, the proportion of asthmatics at treatment step 4 and 5 in ≥1-year group was significantly lower than the <1-year group (48.5% vs. 70.9% and 51.5% vs. 29.1%; P=0.024).
Full table
Full table
Clinical predictors of long-term tiotropium add-on therapy
Univariate and multivariate logistic regression analyses for predictors of long-term tiotropium add-on therapy (≥1-year) were performed. Potential parameters included age, sex, asthma treatment step, ACO, cigarette use, initial FEV1% predicted, BMI and GERD. After univariate analysis, predictors of long-term tiotropium add-on therapy were patients having the following conditions: at asthma treatment step 5 (OR =2.587), ACO (OR =2.599), initial FEV1% predicted <80% (OR =8.230), or BMI >30 kg/m2 (OR =2.776). And in real-world, clinical predictors for patients required long-term tiotropium add-on therapy for asthma control included initial FEV1% predicted <80% (OR =8.429), BMI >30 kg/m2 (OR =4.250), or high proportion of ACO (OR =3.054) using multivariate analysis (Table 2).

Full table
Discussion
The current study indicates that a 3-month tiotropium add-on therapy is beneficial to the maintenance treatment for asthmatics under low- or medium-to-high-dose ICS plus LABA treatment (the ACT score increased from 13.7 to 20.1 points and from 12.6 to 19.2 points in GINA steps 3 and 4 patients, respectively). Several phase III studies already investigated the efficacy and safety of tiotropium as an add-on to maintenance therapy among uncontrolled asthmatics (3,6,7,16,17). None of these studies reported predictors for positive tiotropium response. Liccardi et al. reported the significance of identifying asthmatic subgroups, i.e., who may benefit from tiotropium add-on therapy according to the basal cholinergic tone (18). The authors believed that increased basal cholinergic tone could be a possible predictor of tiotropium response. As finding out tiotropium good responders (TR) and those needed long-term tiotropium add-on therapy in TR group remained unknown, it is to the best of our knowledge that this is the first real-life study characterizing patients requiring long-term tiotropium add-on therapy. Our study results indicated that patients with initial FEV1% predicted <80% (OR =8.43), BMI >30 kg/m2 (OR =4.25), or ACO (OR =3.05) are more likely needing long-term tiotropium add-on therapy.
Asthma can be divided into Th-2-asthma (allergic, eosinophilic, and exercise-induced asthma) and non-Th-2 asthma (smoking, neutrophilic, and obesity-related) (19). Clinical asthma phenotypes not associated with eosinophils included obesity-related late-onset asthma, fixed airway obstruction asthma, asthma-associated neutrophilia, and very few inflammation (20). Our result demonstrated that asthmatics with initial FEV1% predicted <80% may need long-term tiotropium add-on therapy for symptom control. The result was consistent with a previous research, which indicated that tiotropium add-on therapy is effective on patients having positive response to albuterol, airway obstruction and higher cholinergic tone (10). Patients with non-Th-2 driven asthma always have neutrophilic airway inflammation, which is associated with poor response to corticosteroid, severe refractory asthma, lower pre- and post-bronchodilator FEV1, and more air trapping in lung (21,22). Iwamoto et al. also reported that neutrophil sputum profile was positively correlated with the improvement of FEV1 after tiotropium add-on therapy in severe asthmatics (12). Therefore, non-eosinophilic sputum and lower post-bronchodilator FEV1 may be a better predictor for the responses to tiotropium adds-on therapy.
Our study also indicated that obese asthmatic patients (BMI >30 kg/m2) required long-term tiotropium add-on therapy to the maintenance medium-to-high-dose ICS/LABA treatment, suggesting that obese asthmatics may have more severe symptoms and poorer response to corticosteroids (23). In other words, obesity-related asthma may be associated with neutrophilic or paucigranulocytic airway inflammation (24). van Veen et al. reported that obesity in patients with difficult-to-treat asthma was inversely related to sputum eosinophil and exhaled nitric oxide fraction (FeNO) measurements (25). Obese asthmatics had lower total lung capacity, expiratory residual volume and functional residual capacity than non-obese subjects (26). Moreover, dynamic hyperinflation was greater in obese asthmatics with bronchoconstriction (27). Other comorbid conditions, such as GERD and sleep apnea also linked to obesity, and may explain the relationship between increased BMI and the severity of asthma (25). Dixon et al. also pointed out that Th-2 low asthmatics may have airway hyper-responsiveness (AHR) that is directly mediated by obesity-related mechanism, such as increased cholinergic tone and neurogenic inflammation (28). Besides, high serum insulin levels in obese patients can increase vagal-induced bronchoconstriction through acetylcholine release and vagal signaling (29). Based on the above reasons, obese asthmatic patients with ICS/LABA requires adding on another bronchodilator (tiotropium) to achieve asthma control.
Cigarette smoking may induce severe asthma and reduce response to corticosteroid (30). A long-term observational study (over 23-year follow-up) indicated that treatment with ICS in patients with moderate to severe asthma, who had smoked for <5 pack-years, was associated with the reduction in FEV1 declining. However, the therapeutic effect did not show in patients with a history of >5 pack-year smoking (31). In the TENOR study, persistent airflow obstruction in patient with difficult-to-treat asthma is associated with current or past smoking (32). Furthermore, increased cholinergic tone also contributed to airflow obstruction in COPD (33). Patients with asthma and COPD overlap (i.e., ACO) was found to be good responders to anticholinergics, suggesting that anticholinergics could have proportionately greater effect than B2-agonists in COPD patients (34). Current study result was similar to these reports, indicating that patients with ACO might not achieve asthma control with merely ICS/LABA treatment. They required long-term tiotropium add-on therapy to such maintenance treatment to achieve symptomatic asthma control.
Several phases III double-blind, placebo-controlled trials have demonstrated efficacy profiles of adding tiotropium to ICS/LABA treatment in patients with mild to severe asthma (3,6,7), regardless of patient’s baseline characteristics and clinical phenotypes (8,9). Subjects enrolled in these studies were diagnosed of asthma before the age of 40, had no prior smoking history or were ex-smoker with a total of <10 pack-years. The exclusion criteria included the diagnosis of COPD or serious comorbidity. However, in real-world clinical practice, asthmatics smokers always presented with a few comorbidity. Therefore, the core idea of our real-world study is its clinical nature rather than a perfect laboratory study design. To investigate a study with the most appropriate prescriptions closer to the real-world, detailed and meticulous data management is helpful to reduce study biases (35). Out intention of conducting a real-world study is to point out that tiotropium add-on therapy-led improvement of asthma control is independent of clinical characteristics (except for total serum IgE >430 IU/mL) (13), which the results seems to be similar to phase III clinical studies. However, if the application of tiotropium add-on therapy lasts for 6 months or longer, using GINA guidelines as the cut-off point (methods used in the RCTs) is not practical in real-world practice. The current study uses another more trustworthy method, the success of step-down (or withdraw) treatment, to identify subgroups require tiotropium as a maintenance asthma therapy.
There were several limitations in this study. First, it was a single institute observational study involved only a small number of subjects. The patient number between the TGR subgroups (≥1- vs. <1-year) was imbalance due to the lack of control group. This was also a disadvantage of a retrospective observational study. Second, the current study used ACT score as an initial evaluation approach of asthma control 3 months after the add-on therapy of tiotropium, which was different from previous studies (PFT or asthma exacerbation). ACT is a convenient and useful tool in our clinical practice, and we could adjust asthma control medications according to the ACT score. PFT and asthma exacerbation are not practical in real-world clinical practice because PFT is not allowed to perform every 3 months according to the health insurance policy in Taiwan; while asthma exacerbation is not easy to record and detect based on electronic medical record. However, PFT would be performed at least 6 months to one year in the follow-up visit. Third, our study aims to characterize asthma subgroups that require long-term tiotropium add-on to maintenance therapy by stepping down tiotropium after achieving asthma control. We believe that compare to the asthma phenotyping in previous phase III studies, it is more reliable and closer to clinical practice to predict the effectiveness of tiotropium. Fourth, we did not report the ACT score of the TR subgroups (≥1- vs. <1-year) because asthma maintenance treatment in this group was adjusted on a monthly basis mainly according to GINA score. Fifth, cigarette smoking was not clearly defined in our study. Smoking status should be defined as non-smoker, ex-smoker or current smoker including pack-year because it significantly affects bronchial inflammation and epithelial change. Sixth, the study did not report the adherence rate of the patients. However, we believed that the adherence rate between ≥1- and <1-year tiotropium add-on therapy was not significantly different because all asthmatics participated in P4P scheme. Seventh, we did not show more information about Th2 inflammation, such as allergic rhinitis, atopic dermatitis, nasal polyp, FeNO, and serum specific IgE allergy test. Finally, this was not a prospective, randomized controlled study, there might be some bias, confounding factors influencing the results upon analysis. The presented data and proposed predictors should be verified in upcoming large prospective, randomized studies.
Conclusions
Tiotropium add-on therapy may be effective in real-life clinical practice for uncontrolled asthmatics (either low- or medium-to-high ICS plus LABA). Once asthma control had been achieved after tiotropium add-on therapy, a step-down treatment may be required except for asthmatics with ACO, initial FEV1% predicted <80%, or BMI >30 kg/m2. Based on the results, subgroups require long-term tiotropium add-on as a maintenance therapy can be identified. A large prospective study to confirm the results is still warranted in the future.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study has been approved by China Medical University Hospital Institutional Review Board (CMUH103-REC1-112), and written consent was obtained from all patients.
References
- Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011;364:1093-103. [Crossref] [PubMed]
- Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008;359:1543-54. [Crossref] [PubMed]
- Kerstjens HA, Engel M, Dahl R, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med 2012;367:1198-207. [Crossref] [PubMed]
- Kerstjens HA, Disse B, Schroder-Babo W, et al. Tiotropium improves lung function in patients with severe uncontrolled asthma: a randomized controlled trial. J Allergy Clin Immunol 2011;128:308-14. [Crossref] [PubMed]
- Rodrigo GJ, Castro-Rodriguez JA. Tiotropium for the treatment of adolescents with moderate to severe symptomatic asthma: a systematic review with meta-analysis. Ann Allergy Asthma Immunol 2015;115:211-6. [Crossref] [PubMed]
- Kerstjens HA, Casale TB, Bleecker ER, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respir Med 2015;3:367-76. [Crossref] [PubMed]
- Paggiaro P, Halpin DM, Buhl R, et al. The Effect of Tiotropium in Symptomatic Asthma Despite Low- to Medium-Dose Inhaled Corticosteroids: A Randomized Controlled Trial. J Allergy Clin Immunol Pract 2016;4:104-13.e2. [Crossref] [PubMed]
- Kerstjens HA, Moroni-Zentgraf P, Tashkin DP, et al. Tiotropium improves lung function, exacerbation rate, and asthma control, independent of baseline characteristics including age, degree of airway obstruction, and allergic status. Respir Med 2016;117:198-206. [Crossref] [PubMed]
- Casale TB, Bateman ED, Vandewalker M, et al. Tiotropium Respimat Add-on Is Efficacious in Symptomatic Asthma, Independent of T2 Phenotype. J Allergy Clin Immunol Pract 2018;6:923-35.e9. [Crossref] [PubMed]
- Peters SP, Bleecker ER, Kunselman SJ, et al. Predictors of response to tiotropium versus salmeterol in asthmatic adults. J Allergy Clin Immunol 2013;132:1068-74.e1. [Crossref] [PubMed]
- Liccardi G, Salzillo A, Calzetta L, et al. Can an increased cholinergic tone constitute a predictor of positive response to tiotropium in patients with moderate asthma? J Allergy Clin Immunol Pract 2016;4:791-3. [Crossref] [PubMed]
- Iwamoto H, Yokoyama A, Shiota N, et al. Tiotropium bromide is effective for severe asthma with noneosinophilic phenotype. Eur Respir J 2008;31:1379-80. [Crossref] [PubMed]
- Cheng WC, Wu BR, Liao WC, et al. Clinical predictors of the effectiveness of tiotropium in adults with symptomatic asthma: a real-life study. J Thorac Dis 2018;10:3661-9. [Crossref] [PubMed]
- Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol 2004;113:59-65. [Crossref] [PubMed]
- Schatz M, Kosinski M, Yarlas AS, et al. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol 2009;124:719-23.e1. [Crossref] [PubMed]
- Hamelmann E, Bateman ED, Vogelberg C, et al. Tiotropium add-on therapy in adolescents with moderate asthma: A 1-year randomized controlled trial. J Allergy Clin Immunol 2016;138:441-50.e8. [Crossref] [PubMed]
- Vogelberg C, Moroni-Zentgraf P, Leonaviciute-Klimantaviciene M, et al. A randomised dose-ranging study of tiotropium Respimat® in children with symptomatic asthma despite inhaled corticosteroids. Respir Res 2015;16:20. [Crossref] [PubMed]
- Liccardi G, Calzetta L, Salzillo A, et al. Can a better patients' phenotyping predict the efficacy of tiotropium in symptomatic asthma? Allergy Asthma Proc 2017;38:19-20. [Crossref] [PubMed]
- Chari VM, McIvor RA. Tiotropium for the Treatment of Asthma: Patient Selection and Perspectives. Can Respir J 2018;2018:3464960. [Crossref] [PubMed]
- Carr TF, Zeki AA, Kraft M. Eosinophilic and Noneosinophilic Asthma. Am J Respir Crit Care Med 2018;197:22-37. [Crossref] [PubMed]
- Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 2012;18:716-25. [Crossref] [PubMed]
- Fahy JV. Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc 2009;6:256-9. [Crossref] [PubMed]
- Dixon AE, Holguin F, Sood A, et al. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc 2010;7:325-35. [Crossref] [PubMed]
- Sideleva O, Suratt BT, Black KE, et al. Obesity and asthma: an inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med 2012;186:598-605. [Crossref] [PubMed]
- van Veen IH, Ten Brinke A, Sterk PJ, et al. Airway inflammation in obese and nonobese patients with difficult-to-treat asthma. Allergy 2008;63:570-4. [Crossref] [PubMed]
- Lessard A, Turcotte H, Cormier Y, et al. Obesity and asthma: a specific phenotype? Chest 2008;134:317-23. [Crossref] [PubMed]
- Sutherland TJ, Cowan JO, Taylor DR. Dynamic hyperinflation with bronchoconstriction: differences between obese and nonobese women with asthma. Am J Respir Crit Care Med 2008;177:970-5. [Crossref] [PubMed]
- Dixon AE, Pratley RE, Forgione PM, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol 2011;128:508-15.e1-2.
- Leiria LOS, Arantes-Costa FM, Calixto MC, et al. Increased airway reactivity and hyperinsulinemia in obese mice are linked by ERK signaling in brain stem cholinergic neurons. Cell Rep 2015;11:934-43. [Crossref] [PubMed]
- Vonk JM, Jongepier H, Panhuysen CI, et al. Risk factors associated with the presence of irreversible airflow limitation and reduced transfer coefficient in patients with asthma after 26 years of follow up. Thorax 2003;58:322-7. [Crossref] [PubMed]
- Dijkstra A, Vonk JM, Jongepier H, et al. Lung function decline in asthma: association with inhaled corticosteroids, smoking and sex. Thorax 2006;61:105-10. [Crossref] [PubMed]
- Lee JH, Haselkorn T, Borish L, et al. Risk factors associated with persistent airflow limitation in severe or difficult-to-treat asthma: insights from the TENOR study. Chest 2007;132:1882-9. [Crossref] [PubMed]
- Kistemaker LE, Bos IS, Hylkema MN, et al. Muscarinic receptor subtype-specific effects on cigarette smoke-induced inflammation in mice. Eur Respir J 2013;42:1677-88. [Crossref] [PubMed]
- Quirce S, Dominguez-Ortega J, Barranco P. Anticholinergics for treatment of asthma. J Investig Allergol Clin Immunol 2015;25:84-93. [PubMed]
- Kim HS, Lee S, Kim JH. Real-world Evidence versus Randomized Controlled Trial: Clinical Research Based on Electronic Medical Records. J Korean Med Sci 2018;33:e213. [Crossref] [PubMed]


