
Diaphragmatic plication for iatrogenic respiratory insufficiency after cardiothoracic surgery
Introduction
Diaphragmatic paralysis can lead to elevation and loss of mobility of the diaphragm, which may cause atelectasis, mediastinal shift to the contralateral side, and paradoxical movement of the paralyzed diaphragm. When such symptoms do not improve after a certain period of observation or pulmonary rehabilitation, diaphragmatic plication (DP) is considered (1,2).
The efficacy of DP for diaphragmatic paralysis has been investigated in some previous studies and good outcomes have been found (3-7). However, most of those studies included only patients with relatively mild clinical conditions. Very few reports have discussed the value of DP for patients with severe respiratory insufficiency, such as patients who required support from mechanical ventilators (8-10). Although DP for patients with severe respiratory insufficiency is supposed to improve symptoms, the efficacy of the surgery and operative indications remain unclear.
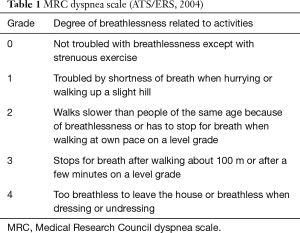
The study aim was to demonstrate the efficacy of DP for patients with severe respiratory insufficiency caused by diaphragmatic paralysis. Therefore, we assessed our experience of DP for patients who continued to experience dyspnea after cardiothoracic surgery with a Medical Research Council (MRC) dyspnea scale (ATS/ERS 2004) (11) score of 4; patient is too breathless to leave the house or breathless when dressing or undressing.
Methods
Between January 2002 and December 2016, a total of 3,312 patients underwent open heart surgery at our institution. We retrospectively examined the clinical data of 10 patients with iatrogenic diaphragmatic paralysis who underwent DP during this period. All the patients underwent fluoroscopy to confirm that the affected diaphragm was elevated and fixed still. Fluoroscopy was conducted in patients connected to mechanical ventilators in the same manner as the other patients with mechanical ventilation interrupted during the exam. The patient’s characteristics, operative procedures, and postoperative outcomes were retrospectively reviewed from our surgical database. We evaluated each patient’s MRC dyspnea scale score (Table 1) and excluded patients whose score was ≤3 because this study was conducted to assess DP in patients with severe respiratory insufficiency due to diaphragmatic paralysis.
Full table
This study was approved by the institutional ethics board of Kobe University Hospital (No. 180061) and written informed consent was obtained from all patients.
Patient characteristics
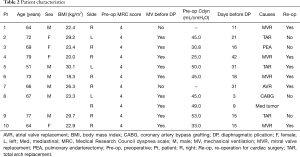
Table 2 shows the clinical characteristics of the patients. The mean age was 68.2 (range, 51–79) years, and 60.0% of the patients were male. Paralysis was right-sided in seven patients, left-sided in two, and bilateral in one patient. The MRC dyspnea scale score of every patient was 4; eight patients required mechanical ventilation, and two patients needed high-flow oxygen therapy prior to DP. They were unable to leave the house or hospitals, which was equivalent to MRC dyspnea scale of 4. The mean length from onset of diaphragmatic paralysis to DP was 19.3 (range, 3–42) days. The cause of diaphragmatic paralysis was phrenic nerve injury after cardiothoracic surgery: four patients following mitral valve replacement, three following total arch replacement (TAR), one following coronary artery bypass graft (CABG), one following pulmonary endarterectomy (PE), and one following mediastinal tumor resection. Among those procedures, 6 (60%) patients underwent cardiovascular re-operation.
Full table
Surgical indication for this study
In our institution, the surgical indications of DP for patients with severe respiratory insufficiency caused by diaphragmatic paralysis include the following: (I) PaO2/FiO2 ratio <200 without mechanical ventilation, (II) existence of paradoxical movement with respiration, and (III) mediastinal shift. We did not perform DP when the respiratory condition was improving as a result of medication and rehabilitation or if considered to be exacerbated by other reasons. For example, we confirmed prior to DP that cardiac and fluid status of each patient did not negatively influence the respiratory insufficiency. If respiratory condition could be improved by their treatment, we waited to perform DP until the treatment was completed.
Surgical procedure
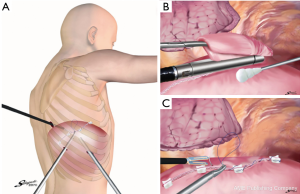
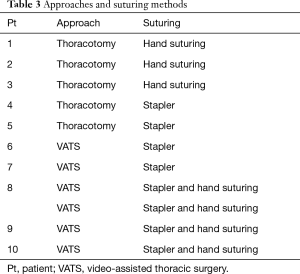
DP was achieved by performing a thoracotomy in the seventh intercostal space with contralateral differential lung ventilation for the first five patients and then with a three-port video-assisted thoracic surgery (VATS) approach (Figure 1A) for the others. Concerning diaphragmatic suturing, a hand-sewn suturing technique was used in the first three patients, the endostapler technique was used in the next four patients, and a combined technique of both hand-suture and an endostapler was used in the other patients (Table 3).
Full table
In the hand-sewn technique, the hemidiaphragm was plicated by using 2-0 polypropylene with an over-and-over suturing technique. After the introduction of endostapler devices, they were used to transect the hemidiaphragm (Figure 1B). However, one out of the 10 patients showed potential vulnerabilities of the staple line treated with the endostapler-only technique. The patient required re-operation 3 days after DP because staple line rupture occurred. Subsequently, a combined technique using hand-suture and the endostapler has been adopted for DP to reinforce suture lines (Figure 1C). During left DP, we cut the diaphragm to allow visualization of the adhesion between the diaphragm and abdominal organs and, thus, avoid damage to the intra-abdominal organs. For right DP, we do not cut diaphragm because the risk of injuring the intestinal tract is much lower than the left side due to position of the liver.
Study procedure
We examined clinical information, including patient characteristics, respiratory status, imaging findings, surgical techniques, interval of mechanical ventilation, length of hospital stay (LOS), postoperative complications, and mortality. Improvements following surgery were assessed by evaluating the position of the hemidiaphragm on chest radiographs, period of time attached to mechanical ventilators, lung dynamic compliance, and postoperative MRC dyspnea scale score; the latter was evaluated 30 days and 90 days after surgery.
Results
Table 4 shows postoperative outcomes. Only one patient, who had undergone the endostapler-only technique, required re-operation because of ruptures at the staple lines, and two patients needed tracheostomy because of a long period of mechanical ventilation. No other postoperative complications were observed, and none of the patients died during the follow-up period. All the patients were successfully withdrawn from mechanical ventilation and discharged without the need for oxygen therapy.
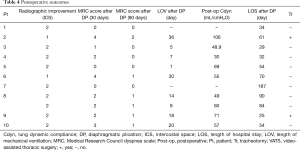
Full table
Postoperative radiography also showed improvement in diaphragmatic elevation and lowering of the central tendons: the mean improvement was 1.8±0.4 intercostal spaces. The MRC dyspnea scale (mean preoperative scale score: 4.0) improved at both 30 and 90 days after surgery, with mean dyspnea scale scores of 1.8±1.4 and 0.6±0.6, respectively. Lung dynamic compliance showed preferable improvement (mean improvement: 41.9±8.9 to 60.7±19.7 mL/cmH2O, normal value: 40–80). The mean length of required mechanical ventilator after surgery was 13.9±11.7 days (range, 0–36 days). The mean LOS following surgery was 65.5±43.8 days (range, 25–187 days).
This study included a patient with bilateral diaphragmatic paralysis (Patient #8) who underwent DP on both sides. Although diaphragm functions did not improve, elevated diaphragms were returned to normal positions on chest radiographs and his MRC dyspnea scales were improved as well as the unilateral cases.
Discussion
Diaphragmatic paralysis in adults is caused by idiopathic factors, tumor invasion, or nerve damage from cardiothoracic surgery (12). Some previous reports have stated that phrenic nerve injury is most commonly caused by mechanical trauma during thoracic tumor resection, cardiac surgery, and internal mammary artery harvesting for CABG (12-17). However, most of our patients had diaphragmatic paralysis after cardiac surgery under severe situations, such as re-operations or emergency surgeries. Considering that patients with diaphragmatic paralysis after resection of thoracic malignancies rarely present with severe respiratory distress, the severity of respiratory conditions in our patients could have been caused by the combined factors of diaphragmatic paralysis, perioperative low cardiac function, and operative stress. Because most of our patients underwent emergency surgery such as TAR and PE, only few patients had taken pulmonary function tests before primary surgery (Data not shown). The patients had a relatively poor pulmonary function at baseline and we consider that they did not tolerate diaphragmatic paralysis partly because of their preoperative low respiratory function.
The operative indications and timing of DP for patients with severe conditions due to diaphragmatic paralysis remain controversial because most patients are able to improve their symptoms by conservative observation or rehabilitation, and complete recovery of diaphragmatic function would not be expected if DP was performed (13,18,19) in such cases. Diaphragmatic function has been found to return to normal within 6 to 12 months after traumatic phrenic nerve injury (20). Therefore, some previous reports have recommended that surgery should be performed 6 to 12 months after phrenic nerve injury (1,5,7,19,21). However, we could not delay the surgery for such a long period because our patients had severe respiratory insufficiency, and long-term observation could lead to major complications, such as pneumonia, ventilator-induced lung injury, or muscle weakness. A few previous case reports have shown that early surgical intervention contributed to good outcomes in patients with severe respiratory distress (8,22), whereas other reports have shown that surgical intervention performed >6 weeks after diaphragmatic paralysis led to poor outcomes for mechanically ventilated adult patients with severe lung parenchymal disease (10). Additionally, late DP might result in poor outcomes because of atrophy of the diaphragm (14). Consequently, we established surgical criteria for diaphragmatic paralysis with severe respiratory insufficiency in our institution and performed emergency surgeries. Although some patients required long LOS, respiratory conditions improved in all of our patients. Two patients (Patients #2, #6) took more than 30 days from DP to extubation, and there were no preoperative factors for prolonged mechanical ventilation such as cardiac function, frailty, or BMI.
We consider that patient selection is important for good outcomes; therefore, we do not perform DP when the respiratory condition is improving in response to medication and rehabilitation or considered to be exacerbated for other reasons such as overhydration or cardiac failure. All of our patients had undergone echocardiography to rule out other potential factors of respiratory insufficiency. If respiratory condition could be improved by their treatment, we waited to perform DP until the treatment was completed.
In fact, we could not grasp the accurate number of patients who developed iatrogenic diaphragmatic paralysis during the period because patients who did not undergo DP were not obtained from our database. However, about half of the cases who had respiratory insufficiency and were considered for DP showed some respiratory improvement in a short duration and did not require DP. It is assumed that improvement of other factors than diaphragmatic paralysis led to the improvement of their respiratory conditions. From our results, we considered that early surgical intervention contributed to good outcomes in such patients with severe respiratory insufficiency under careful patient selection.
Several previous studies have described the use of thoracoscopic DP and reported good outcomes (23-25). However, the advantages of VATS over thoracotomy for DP have been less frequently reported (24). We believe that the most important advantage of VATS for patients is the preservation of respiratory muscles. VATS lobectomy reportedly prevents atrophy of the latissimus dorsi muscles, which allows for faster recovery of short-term respiratory function (26-28). We assume that the preservation of respiratory muscles is more important in patients with diaphragmatic dysfunction than in patients who underwent lung lobectomy because the role of respiratory muscles is greater in patients with diaphragmatic dysfunction. Moreover, we consider that short-term recovery is an additional advantage for our patients.
Some suturing methods of DP have been reported previously. However, no studies have evaluated the association between suturing methods and their outcomes (29,30). We consider that resection of a redundant diaphragm contributed to good outcomes regardless of the suturing method. Actually, our patients with poor improvement in diaphragmatic elevation needed a tracheotomy and longer periods of mechanical ventilation. Of course, the more the number of intercostal spaces the diaphragm is lowered for, the more the respiratory function would improve. However, there was no suggestion about the target number of intercostal spaces to be reached during the repair. Our patients showed improvement of elevated diaphragm by 1.8±0.4 intercostal spaces on average, and all of them showed improvement in the MRC dyspnea scale. This suggests that we can set a target number of intercostal spaces of at least two in the future.
Although conventional hand suturing is the most secure and durable method for DP, it is time-consuming and complicated when used in the VATS procedure. We think the endostapler device is particularly useful for the VATS procedure and saves time and labor. We currently perform DP with a combination of an endostapler and hand-suture methods because we experienced a case of rupture at the staple lines in Patient #7 who was treated with the endostapler-only technique. Indeed, some reports have recommended reinforcement of the staple line with sutures to avoid ruptures (23,31). We found that this combined technique was convenient for VATS because suturing after the redundant diaphragm had been removed was easier than using hand sutures only.
There were three major limitations in this study: (I) the sample size was small, so further studies are required with a larger number of patients; (II) few objective examination data were obtained. Most of our patients were dependent on mechanical ventilation, which made it difficult to assess improvement in their respiratory function. Therefore, we used improvement of diaphragm elevation in chest radiography and lung dynamic compliance instead. However, new objective methods of assessment should be considered prior to surgery to determine the surgical indicators for DP in patients with severe respiratory insufficiency; (III) the denominator of patients with iatrogenic diaphragmatic paralysis was not obtained in this study. With the accurate number of patients with iatrogenic diaphragmatic paralysis, including both who did and did not undergo DP, we could understand more of the association between iatrogenic diaphragmatic paralysis and indication of DP. Further studies would help us establish surgical indication of DP for severe diaphragmatic paralysis.
In conclusion, performing DP for patients with severe respiratory insufficiency caused by diaphragmatic paralysis after cardiothoracic surgery contributed to improvement in dyspnea. Early surgical intervention could be considered for these patients if their respiratory condition is not expected to improve or is believed to be influenced by other factors.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional ethics board of Kobe University Hospital (No. 180061) and written informed consent was obtained from all patients. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Freeman RK, Van Woerkom J, Vyverberg A, et al. Long-term follow-up of the functional and physiologic results of diaphragm plication in adults with unilateral diaphragm paralysis. Ann Thorac Surg 2009;88:1112-7. [Crossref] [PubMed]
- Tokunaga T, Sawabata N, Kadota Y, et al. Efficacy of intra-operative unilateral diaphragm plication for patients undergoing unilateral phrenicotomy during extended surgery. Eur J Cardiothorac Surg 2010;38:600-3. [Crossref] [PubMed]
- Stamenovic D. New technique of diaphragmatic plication by means of uniportal video-assisted thoracoscopic surgery. Interact Cardiovasc Thorac Surg 2017;25:162-3. [Crossref] [PubMed]
- Higgs SM, Hussain A, Jackson M, et al. Long term results of diaphragmatic plication for unilateral diaphragm paralysis. Eur J Cardiothorac Surg 2002;21:294-7. [Crossref] [PubMed]
- Versteegh MI, Braun J, Voigt PG, et al. Diaphragm plication in adult patients with diaphragm paralysis leads to long-term improvement of pulmonary function and level of dyspnea. Eur J Cardiothorac Surg 2007;32:449-56. [Crossref] [PubMed]
- Calvinho P, Bastos C, Bernardo JE, et al. Diaphragmmatic eventration: long-term follow-up and results of open-chest plicature. Eur J Cardiothorac Surg 2009;36:883-7. [Crossref] [PubMed]
- Celik S, Celik M, Aydemir B, et al. Long-term results of diaphragmatic plication in adults with unilateral diaphragm paralysis. J Cardiothorac Surg 2010;5:111. [Crossref] [PubMed]
- Glassman LR, Spencer FC, Baumann FG, et al. Successful plication for postoperative diaphragmatic paralysis in an adult. Ann Thorac Surg 1994;58:1754-5; discussion 1757-8.
- Kuniyoshi Y, Yamashiro S, Miyagi K, et al. Diaphragmatic plication in adult patients with diaphragm paralysis after cardiac surgery. Ann Thorac Cardiovasc Surg 2004;10:160-6. [PubMed]
- Simansky DA, Paley M, Refaely Y, et al. Diaphragm plication following phrenic nerve injury: a comparison of paediatric and adult patients. Thorax 2002;57:613-6. [Crossref] [PubMed]
- Celli BR, MacNee W, Force AET. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932-46. [Crossref] [PubMed]
- Groth SS, Andrade RS. Diaphragm plication for eventration or paralysis: a review of the literature. Ann Thorac Surg 2010;89:S2146-50. [Crossref] [PubMed]
- Deng Y, Byth K, Paterson HS. Phrenic nerve injury associated with high free right internal mammary artery harvesting. Ann Thorac Surg 2003;76:459-63. [Crossref] [PubMed]
- Aguirre VJ, Sinha P, Zimmet A, et al. Phrenic nerve injury during cardiac surgery: mechanisms, management and prevention. Heart Lung Circ 2013;22:895-902. [Crossref] [PubMed]
- Tripp HF, Sees DW, Lisagor PG, et al. Is phrenic nerve dysfunction after cardiac surgery related to internal mammary harvesting? J Card Surg 2001;16:228-31. [Crossref] [PubMed]
- Ariyoshi T, Hashizume K, Taniguchi S, et al. Surgical experience with chronic constrictive pericarditis. Gen Thorac Cardiovasc Surg 2012;60:796-802. [Crossref] [PubMed]
- Hirata Y, Hirahara N, Murakami A, et al. Current status of cardiovascular surgery in Japan 2013 and 2014: A report based on the Japan Cardiovascular Surgery Database. 2: Congenital heart surgery. Gen Thorac Cardiovasc Surg 2018;66:4-7. [Crossref] [PubMed]
- Summerhill EM, El-Sameed YA, Glidden TJ, et al. Monitoring recovery from diaphragm paralysis with ultrasound. Chest 2008;133:737-43. [Crossref] [PubMed]
- Kodric M, Trevisan R, Torregiani C, et al. Inspiratory muscle training for diaphragm dysfunction after cardiac surgery. J Thorac Cardiovasc Surg 2013;145:819-23. [Crossref] [PubMed]
- Iverson LI, Mittal A, Dugan DJ, et al. Injuries to the phrenic nerve resulting in diaphragmatic paralysis with special reference to stretch trauma. Am J Surg 1976;132:263-9. [Crossref] [PubMed]
- Freeman RK, Wozniak TC, Fitzgerald EB. Functional and physiologic results of video-assisted thoracoscopic diaphragm plication in adult patients with unilateral diaphragm paralysis. Ann Thorac Surg 2006;81:1853-7; discussion 1857.
- Tsakiridis K, Visouli AN, Zarogoulidis P, et al. Early hemi-diaphragmatic plication through a video assisted mini-thoracotomy in postcardiotomy phrenic nerve paresis. J Thorac Dis 2012;4 Suppl 1:56-68. [PubMed]
- Kocher GJ, Zehnder A, Schmid RA. Completely Thoracoscopic Diaphragmatic Plication. World J Surg 2017;41:1019-22. [Crossref] [PubMed]
- Gazala S, Hunt I, Bédard EL. Diaphragmatic plication offers functional improvement in dyspnoea and better pulmonary function with low morbidity. Interact Cardiovasc Thorac Surg 2012;15:505-8. [Crossref] [PubMed]
- Dunning J. Thoracoscopic diaphragm plication. Interact Cardiovasc Thorac Surg 2015;20:689-90. [Crossref] [PubMed]
- Karasaki T, Nakajima J, Murakawa T, et al. Video-assisted thoracic surgery lobectomy preserves more latissimus dorsi muscle than conventional surgery. Interact Cardiovasc Thorac Surg 2009;8:316-9; discussion 319-20. [Crossref] [PubMed]
- Pu Q, Ma L, Mei J, et al. Video-assisted thoracoscopic surgery versus posterolateral thoracotomy lobectomy: A more patient-friendly approach on postoperative pain, pulmonary function and shoulder function. Thorac Cancer 2013;4:84-9. [Crossref] [PubMed]
- Nakata M, Saeki H, Yokoyama N, et al. Pulmonary function after lobectomy: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2000;70:938-41. [Crossref] [PubMed]
- Groth SS, Rueth NM, Kast T, et al. Laparoscopic diaphragmatic plication for diaphragmatic paralysis and eventration: an objective evaluation of short-term and midterm results. J Thorac Cardiovasc Surg 2010;139:1452-6. [Crossref] [PubMed]
- Hüttl TP, Wichmann MW, Reichart B, et al. Laparoscopic diaphragmatic plication: long-term results of a novel surgical technique for postoperative phrenic nerve palsy. Surg Endosc 2004;18:547-51. [Crossref] [PubMed]
- Kara HV, Roach MJ, Balderson SS, et al. Thoracoscopic diaphragm plication. Ann Cardiothorac Surg 2015;4:573-5. [PubMed]


