
HIV and tuberculosis co-infection in East Asia and the Pacific from 1990 to 2017: results from the Global Burden of Disease Study 2017
Introduction
Both HIV/AIDS and tuberculosis (TB) are leading causes of morbidity and mortality globally (1,2). In 2017, there were 8,965,800 new cases, 1,929,208,600 prevalent cases, and 1,183,700 deaths of TB, as well as 1,942,100 new cases, 36,822,200 prevalent cases, and 954,500 deaths of HIV/AIDS. Among all HIV/AIDS deaths, nearly 23% are related to TB (1,2). HIV/AIDS, TB, and their co-infection are major global health concerns and have drawn the focus of the United Nations which made Sustainable Development Goal 3.3 to end HIV/AIDS epidemics by 2030. Continued surveillance is crucial in monitoring disease control efforts for both HIV/AIDS and TB. However, there has been insufficient attention placed on HIV/AIDS and TB co-infection in previous disease-specific research such as the Global Burden of Disease (GBD) Study (3-5).
When implementing control efforts for HIV/AIDS and TB, the East Asia and Pacific World Bank region plays an important role due to its high disease burden, particularly of TB (1-5). China and Indonesia, for example, are among the top ten countries in the world with the most prevalent cases of HIV/AIDS and are both top three countries worldwide with the most prevalent cases of TB in 2017 (6). As a region with high disease burdens of both HIV/AIDS and TB, the East Asia and Pacific region is also a high-risk region for their co-infection. In this article, we examined morbidity and mortality of HIV and TB co-infection in the East Asia and Pacific region from 1990 to 2017.
Methods
This study is based on the data and measures of the GBD Study 2017. In brief, the GBD Study series provide comprehensive global disease information by quantifying geographically-specific disease measures such as incidence, prevalence, and mortality, as well as disease burden attributable to risk factors by age, sex, and geography over time. The current study includes data collected from 195 countries and territories from 1990 to 2017. Detailed information about the conception, design, data collection, and data synthesis of the GBD Study 2017 has been published elsewhere (1,2,7-11), and related information for HIV/AIDS, TB, and their co-infection has been described previously as well (3-5).
In this study, HIV and TB co-infection includes: HIV-infected drug-susceptible tuberculosis (HIV-infected DS-TB), HIV-infected multidrug-resistant tuberculosis without extensive drug resistance (HIV-infected MDR-TB without XDR), and HIV-infected extensively drug-resistant tuberculosis (HIV-infected XDR-TB). We examined both-sex incidence, prevalence, and deaths of HIV and TB co-infection based on all-age number and age-standardized rate by three co-infection types from the GBD Study 2017 data. We analyzed the trend of the three above measures for the period between 1990 and 2017 by using a join-point regression model (version 4.6.0.0); a significant trend is indicated when the P value is lower than 0.05 (12,13). For HIV-infected DS-TB, the co-infection type with the highest burden, we conducted trend analysis for each measure (prevalence, incidence, and mortality) in the three countries with the highest number and the highest rate of that measure in 2017 (e.g., trend analysis for HIV-infected DS-TB incidence rate was conducted in Indonesia, Laos, and Myanmar, because their incidence rates of HIV-infected DS-TB were highest among all countries in the East Asia and Pacific region in 2017).
Results
Incidence, prevalence, and mortality of HIV and tuberculosis co-infection in 2017
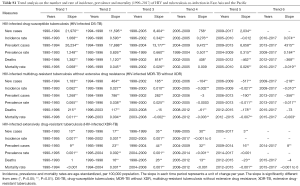
Both-sex, all-age new cases, prevalent cases, and deaths of HIV-infected DS-TB, HIV-infected MDR-TB without XDR, and HIV-infected XDR-TB by countries in East Asia and the Pacific in 2017 are summarized in Table 1. In 2017, there were 238,372, 4,294, and 392 new cases of HIV-infected DS-TB, HIV-infected MDR-TB without XDR, and HIV-infected XDR-TB, respectively. The number of prevalent cases and deaths were 383,809 and 12,197 of HIV-infected DS-TB, 7,811 and 1,168 of HIV-infected MDR-TB without XDR, and 713 and 282 of HIV-infected XDR-TB.
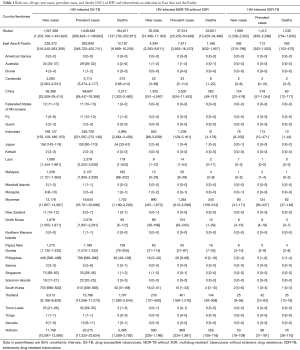
Full table
For HIV-infected DS-TB in 2017, the countries with the highest number of new cases were Indonesia (168,147 cases), China (26,388 cases), and Myanmar (13,178 cases). The countries with the highest number of prevalent cases were Indonesia (246,729 cases), China (68,607 cases), and Vietnam (20,275 cases); and those with the highest number of deaths were Indonesia (2,994 deaths), Vietnam (2,485 deaths), and China (2,212 deaths).
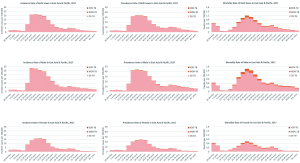
Both-sex, age-standardized incidence rate, prevalence rate and mortality rate (per 100,000 population) of the three HIV and TB co-infection types by countries in East Asia and the Pacific are summarized in Table 2. The age distribution of these three rates are shown for both-sex, male-only, and female-only in Figure 1. For HIV-infected DS-TB in 2017, the top three countries with the highest incidence rate were Indonesia (61.27 new cases per 100,000), Laos (25.55 new cases per 100,000), and Myanmar (23.95 new cases per 100,000); those with the highest prevalence rate were also Indonesia (89.76 prevalent cases per 100,000), Laos (39.00 prevalent cases per 100,000), and Myanmar (33.88 prevalent cases per 100,000); and those with the highest mortality rate were Myanmar (3.08 deaths per 100,000), Vietnam (2.23 deaths per 100,000), and Thailand (1.97 deaths per 100,000).

Full table
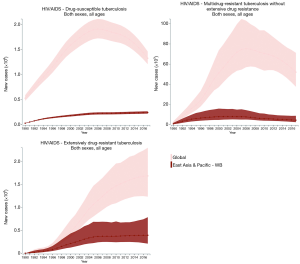
Incidence, prevalence, and mortality trends of HIV and tuberculosis co-infection from 1990 to 2017
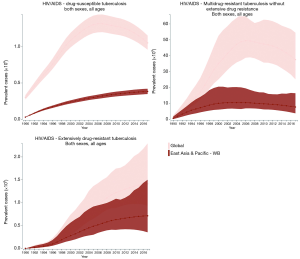
The incidence, prevalence, and mortality trends of HIV and tuberculosis co-infection are shown based on number (Figures S1-S3) and rate (per 100,000 population; Figures 2-4) for the period between 1990 and 2017. These figures show both of global trends and trends in the East Asia and Pacific region. The results from trend analysis are summarized in Table S1. Overall, the incidence rates of HIV-infected DS-TB and HIV-infected MDR-TB without XDR (Figure 2), the prevalence rate of HIV-infected MDR-TB without XDR (Figure 3), and the mortality rate of all three co-infection types (Figure 4) in East Asia and the Pacific were lower than the global rates for the past decade.
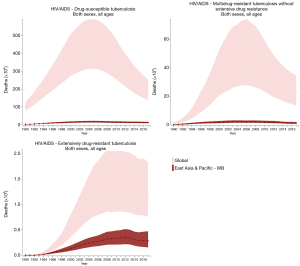
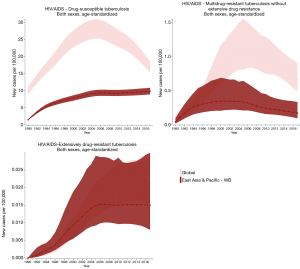
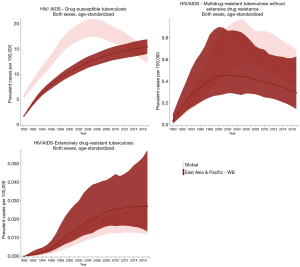
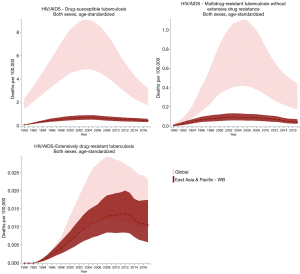
Full table
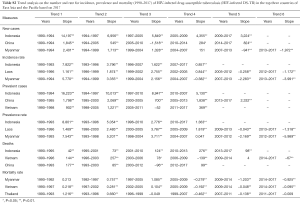
For HIV-infected DS-TB, the overall number and rate for incidence and prevalence have increased from 1990 to 2017 (Table S1). More specifically, the prevalence rate of HIV-infected DS-TB was higher than the global level in recent years (2015–2017) (Figure 3). As for mortality, a significant increase began in 1990 (number: 2,156 deaths; rate: 0.12 deaths per 100,000) and reached its peak in 2005 (number: 17,284 deaths; rate: 0.73 deaths per 100,000); since 2005, mortality has significantly decreased (number in 2017: 12,197 deaths; rate in 2017: 0.47 deaths per 100,000) (Table S1). The countries with the highest value of each disease measure (number and rate) in 2017 are shown in Table S2; most demonstrated a decreasing trend in the three most recent years.
Full table
Each measure for HIV-infected MDR-TB without XDR showed a significant increase followed by a significant decrease after reaching a peak (Table S1). For example, the mortality rate of HIV-infected MDR-TB significantly increased from 0.0067 deaths per 100,000 in 1990, reached its peak (0.09 deaths per 100,000) in 2003, and then significantly decreased to 0.04 deaths per 100,000 in 2017.
The number of HIV-infected XDR-TB incident and prevalent cases as well as prevalence rate presented a continuous increase throughout the period (1990–2017); the incidence rate increased significantly from 1990 to 2005 and then became steady (2005–2017). The mortality rate showed a continuous significant increase and then a significant decrease after reaching a peak in 2012 (Table S1).
Discussion
Complete, timely, reliable, and valid surveillance is important to quantify the morbidity and mortality of HIV/AIDS and TB co-infection for disease control (14-17). This study provides up-to-date measure information including tendency of the co-infection in East Asia and Pacific by utilizing data and measures compiled by the GBD Study 2017. We found that both-sex, age-standardized mortality rates of the three HIV/AIDS and TB co-infection types in East Asia and the Pacific were lower than global rates over the past decade. In addition, the trend in mortality (number and rate) in all co-infection types significantly decreased over the five most recent years of data. However, both the number and rate for incidence and prevalence of HIV-infected DS-TB and HIV-infected XDR-TB have been increasing throughout the period (1990–2017).
The decreasing tendency of mortality demonstrates a remarkable progress in disease treatment. Since the introduction of antiretroviral therapy (ART), a lower mortality rate has been successfully achieved in HIV patients with and without TB (18-25). However, considering the co-infection of both diseases, especially HIV-infected MDR-TB (including HIV-infected XDR-TB), treatment management is more complicated compared to the management of either disease on its own. The complication includes drug interactions, overlapping toxicity, and the possibility of immune reconstitution inflammatory syndrome (IRIS), which may potentially lead to drug resistance, nonadherence to therapy, and/or virological failure (26). To some extent, this complication explains why both the incidence rate and prevalence rate of HIV-infected XDR-TB have not declined over the last decade.
The increasing numbers and rates of incidence and prevalence of HIV-infected DS-TB and HIV-infected XDR-TB reflect the difficulty of prevention efforts. For example, the breakthrough discovery of highly active ART is able to reduce the size of the immunocompromised population and decrease the life-time risk of developing active TB (14). As a result, the World Health Organization (WHO) recommends an earlier initiation of ART to prevent active tuberculosis in people with HIV (27). However, the availability and distribution of these therapies vary—not all HIV patients receive the therapy, regardless of TB status (14,28). According to the GBD Study 2015, the global ART coverage per 100 people living with HIV in 2015 was 40.6%, and the coverage in the high sociodemographic index (SDI) countries was 51.5%. However, the coverage in Southeast Asia, East Asia, and Oceania (major parts of the East Asia and Pacific region) was only 25.9%, lower than not only global coverage but also lower than coverage in low SDI countries (37.9%) (4).
To eliminate both HIV/AIDS and TB, as well as their co-infection, further efforts for treatment are needed. Main strategies should be: early use of ART, early testing to detect drug resistance, sustained adherence to appropriate drug-resistant TB regimens, and proper monitoring for treatment response and adverse effects (17). Early use of ART has been difficult to implement due to low ART coverage, reflecting the limited available resources globally (28,29) and in East Asia and the Pacific, especially in regions with the highest disease morbidity and mortality such as Myanmar (30) and some regions of China (e.g., Xinjiang Province) (31). As for early detection of drug resistance, although a rapid detection of TB and drug resistance in patients living with HIV has been well established (e.g., Xpert MTB/RIF) (32), one major barrier for a broader implementation is the cost (33,34). To achieve sustained adherence to TB regimens and proper monitoring for treatment response and adverse effects, programmatic management is recommended. The Cambodian National MDR-TB program is one example of a successful program that achieved treatment success based on a community-based effort (35).
To achieve the above treatment strategies, main barriers to disease control should be targeted. In addition to limited resources (e.g., ART coverage, cost, well-established programmatic management), efforts should also focus on stigma. Stigma is a substantial challenge for HIV diagnosis and treatment (36-39). This increases the risk of developing TB, and the risk of new HIV/AIDS (and, consequentially, TB) cases through infection. Reducing stigma requires respectful and targeted engagement, especially for marginalized populations [sex workers, men who have sex with men (MSM), transgender people, people who inject drugs, and people in prison] (40). For this, legal and social environments play an important role, especially those involving health professionals (40,41). Incarceration is another major obstacle. According to a systematic review and meta-analysis, the prevalence of HIV/AIDS and TB co-infection among incarcerated individuals is high worldwide: 32.6% globally, and 35% in Asia (42). One reason accounting for such a high prevalence is that incarceration is a main risk factor of developing TB, especially among those who contracted HIV/AIDS via drug injection (43,44). Other reasons include limited resources for diagnosis and treatment adherence, low treatment completion rate due to limited resources as well as movement by individuals into and out of the prison system (42,45). In addition to the above barriers, an evidence gap between research outputs and policy also exists. A study in Cambodia found that only a small proportion of TB research outputs addressed policy and program planning (46). This study indicates substantial room for improvement in alignment between researchers, funders, and policy makers.
This study has several limitations. First, we followed the GBD study methods using aggregated data from the GBD website. Detailed limitations on the GBD study’s methods have been well discussed in pervious publications (1-11). Second, the distribution of HIV/AIDS and TB co-infection is heterogeneous between local regions within each country and the present results using GBD Study measures only demonstrate a country-level picture of the co-infection in East Asia and the Pacific. Furthermore, we did not provide estimates of the co-infection by age and sex for each country. However, detailed estimates are available on the GBD website (http://ghdx.healthdata.org/gbd-results-tool). For further improvement of our future research, it is reasonable to consider those measures among more vulnerable populations, including incarcerated populations.
Conclusions
HIV/AIDS, tuberculosis, and their co-infection constitute a substantial portion of global morbidity and mortality. Our study indicates that the trends in almost all measures of the three co-infection types examined here have been decreasing globally, and their trends in mortality have also been decreasing in East Asia and the Pacific. However, the overall trends in both incidence and prevalence of HIV-infected DS-TB and HIV-infected XDR-TB have been increasing since 1990. Continuous efforts should be kept in progress for disease control. For example, further development of treatments specifically for co-infection and management protocols, as well as cost containment, are needed to reverse the current increasing trends of these co-infection types.
Acknowledgments
None.
Footnote
Conflicts of Interest: This study has been presented at the ATS 2019 International Conference (Dallas, USA).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789-858. [Crossref] [PubMed]
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736-88. [Crossref] [PubMed]
- Murray CJ, Ortblad KF, Guinovart C, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:1005-70. Erratum in: Lancet 2014 Oct 25;384(9953):1504. Lancet 2014;384:956. [Crossref] [PubMed]
- GBD 2015 HIV Collaborators. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980-2015: the Global Burden of Disease Study 2015. Lancet HIV 2016;3:e361-87. [Crossref] [PubMed]
- Tuberculosis Collaborators GBD. The global burden of tuberculosis: results from the Global Burden of Disease Study 2015. Lancet Infect Dis 2018;18:261-84. [Crossref] [PubMed]
- Global Burden of Disease (GBD) Study. Data visualizations. Available online: (accessed on Dec 31, 2018)http://www.healthdata.org/results/data-visualizations
- GBD 2017 SDG Collaborators. Measuring progress from 1990 to 2017 and projecting attainment to 2030 of the health-related Sustainable Development Goals for 195 countries and territories: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:2091-2138. [Crossref] [PubMed]
- GBD 2017 Population and Fertility Collaborators. Population and fertility by age and sex for 195 countries and territories, 1950-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1995-2051. [Crossref] [PubMed]
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1923-94. [Crossref] [PubMed]
- GBD 2017 Mortality Collaborators. Global, regional, and national age-sex-specific mortality and life expectancy, 1950-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1684-735. [Crossref] [PubMed]
- GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1859-922. [Crossref] [PubMed]
- Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335-51. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- van der Werf MJ, Sotgiu G, Dara M. Closing the gap in surveillance of tuberculosis and HIV co-infection: a European perspective on the need for clinician-public health alliances. Eur Respir J 2017. [Crossref] [PubMed]
- de Vries G, van den Hof S, Op de Coul E, et al. Closing the gap in surveillance of tuberculosis and HIV co-infection, and the need for clinician-public health alliances. Eur Respir J 2018. [Crossref] [PubMed]
- Tornheim JA, Dooley KE. Challenges of TB and HIV co-treatment: updates and insights. Curr Opin HIV AIDS 2018;13:486-91. [Crossref] [PubMed]
- Hurtado RM, Meressa D, Goldfeld AE. Treatment of drug-resistant tuberculosis among people living with HIV. Curr Opin HIV AIDS 2018;13:478-85. [Crossref] [PubMed]
- Mutembo S, Mutanga JN, Musokotwane K, et al. Antiretroviral therapy improves survival among TB-HIV co-infected patients who have CD4+ T-cell count above 350cells/mm(3). BMC Infect Dis 2016;16:572. [Crossref] [PubMed]
- Uthman OA, Okwundu C, Gbenga K, et al. Optimal Timing of Antiretroviral Therapy Initiation for HIV-Infected Adults With Newly Diagnosed Pulmonary Tuberculosis: A Systematic Review and Meta-analysis. Ann Intern Med 2015;163:32-9. [Crossref] [PubMed]
- Stockdale AJ, Nkuranga J, Török ME, et al. Initiation of antiretroviral therapy in HIV-infected tuberculosis patients in rural Kenya: an observational study. Trop Med Int Health 2013;18:907-14. [Crossref] [PubMed]
- Abdool Karim SS, Naidoo K, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med 2011;365:1492-501. [Crossref] [PubMed]
- Blanc FX, Sok T, Laureillard D, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med 2011;365:1471-81. [Crossref] [PubMed]
- Franke MF, Robins JM, Mugabo J, et al. Effectiveness of early antiretroviral therapy initiation to improve survival among HIV-infected adults with tuberculosis: a retrospective cohort study. PLoS Med 2011;8:e1001029. [Crossref] [PubMed]
- Velasco M, Castilla V, Sanz J, et al. Effect of simultaneous use of highly active antiretroviral therapy on survival of HIV patients with tuberculosis. J Acquir Immune Defic Syndr 2009;50:148-52. [Crossref] [PubMed]
- Manosuthi W, Chottanapand S, Thongyen S, et al. Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. J Acquir Immune Defic Syndr 2006;43:42-6. [Crossref] [PubMed]
- Egelund EF, Dupree L, Huesgen E, et al. The pharmacological challenges of treating tuberculosis and HIV coinfections. Expert Rev Clin Pharmacol 2017;10:213-23. [Crossref] [PubMed]
- World Health Organization (WHO). WHO Policy on Collaborative TB/HIV Activities: Guidelines for National Programmes and Other Stakeholders. 2012. Available online: (accessed on Jan 11, 2019)http://apps.who.int/iris/bitstream/handle/10665/44789/9789241503006_eng.pdf?sequence=1
- Joulaei H, Shooshtarian S, Dianatinasab M. Is UNAIDS 90-90-90 target a Dream or a Reality for Middle East and North Africa Region on Ending the AIDS Epidemic? A Review Study. AIDS Rev 2018;20:83-93. [PubMed]
- Brown AE, Hayes R, Noori T, et al. HIV in Europe and Central Asia: progress in 2018 towards meeting the UNAIDS 90-90-90 targets. Euro Surveill 2018. [Crossref] [PubMed]
- Kyi MS, Aung ST, McNeil E, et al. Evolution of Tuberculosis/Human Immunodeficiency Virus Services among Different Integrated Models in Myanmar: A Health Services Review. Trop Med Infect Dis 2018. doi: . [Crossref]
- Maimaiti R, Zhang Y, Pan K, et al. High prevalence and low cure rate of tuberculosis among patients with HIV in Xinjiang, China. BMC Infect Dis 2017;17:15. [Crossref] [PubMed]
- Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010;363:1005-15. [Crossref] [PubMed]
- Wang G, Wang S, Jiang G, et al. Incremental cost-effectiveness of the second Xpert MTB/RIF assay to detect Mycobacterium tuberculosis. J Thorac Dis 2018;10:1689-95. [Crossref] [PubMed]
- Khaparde S, Raizada N, Nair SA, et al. Scaling-up the Xpert MTB/RIF assay for the detection of tuberculosis and rifampicin resistance in India: An economic analysis. PLoS One 2017;12:e0184270. [Crossref] [PubMed]
- Sam S, Shapiro AE, Sok T, et al. Initiation, scale-up and outcomes of the Cambodian National MDR-TB programme 2006 – 2016: hospital and community-based treatment through an NGO-NTP partnership. BMJ Open Respir Res 2018;5:e000256. [Crossref] [PubMed]
- Gesesew HA, Tesfay Gebremedhin A, et al. Significant association between perceived HIV related stigma and late presentation for HIV/AIDS care in low and middle-income countries: A systematic review and meta-analysis. PLoS One 2017;12:e0173928. [Crossref] [PubMed]
- Eaton LA, Driffin DD, Kegler C, et al. The role of stigma and medical mistrust in the routine health care engagement of black men who have sex with men. Am J Public Health 2015;105:e75-82. [Crossref] [PubMed]
- Fortenberry JD, McFarlane M, Bleakley A, et al. Relationships of stigma and shame to gonorrhea and HIV screening. Am J Public Health 2002;92:378-81. [Crossref] [PubMed]
- Wei C, Cheung DH, Yan H, et al. The Impact of Homophobia and HIV Stigma on HIV Testing Uptake Among Chinese Men Who Have Sex With Men: a Mediation Analysis. J Acquir Immune Defic Syndr 2016;71:87-93. [Crossref] [PubMed]
- HIV. science and stigma. Lancet 2014;384:207. [Crossref] [PubMed]
- Li L, Wu Z, Zhao Y, et al. Using case vignettes to measure HIV-related stigma among health professionals in China. Int J Epidemiol 2007;36:178-84. [Crossref] [PubMed]
- Dianatinasab M, Joulaei H, Ghorbani M, et al. Prevalence of Tuberculosis in HIV-positive Prisoners: A Systematic Review and Meta-analysis. AIDS Rev 2018;20:114-124. [PubMed]
- Molaeipoor L, Poorolajal J, Mohraz M, et al. Predictors of tuberculosis and human immunodeficiency virus co-infection: a case-control study. Epidemiol Health 2014;36:e2014024. [Crossref] [PubMed]
- Hermosilla S, El-Bassel N, Aifah A, et al. Tuberculosis report among injection drug users and their partners in kazakhstan. Public Health 2015;129:569-75. [Crossref] [PubMed]
- Séri B, Koffi A, Danel C, et al. Prevalence of pulmonary tuberculosis among prison inmates: A cross-sectional survey at the Correctional and Detention Facility of Abidjan, Côte d'Ivoire. PLoS One 2017;12:e0181995. [Crossref] [PubMed]
- Boudarene L, James R, Coker R, et al. Are scientific research outputs aligned with national policy makers' priorities? A case study of tuberculosis in Cambodia. Health Policy Plan 2017;32:i3-i11. [Crossref] [PubMed]


