Analysis of the molecular and clinicopathologic features of surgically resected lung adenocarcinoma in patients under 40 years old
Introduction
Lung cancer, one of the most frequent malignancies in the world, is rapidly becoming the main cause of cancer-related death nowadays (1). During the past decade, its incidence has been quickly increasing and age at diagnosis keeps decreasing (2,3). Many studies have suggested that lung cancer in young patients may constitute an entity with distinct clinicopathologic characteristics, which is common in non-smoker and female patients, and presents a predominance of adenocarcinoma, the advanced stage at diagnosis and thus a generally poor prognosis (4,5). However, it is still controversial whether younger patients with lung cancer have better or worse outcomes compared with the older counterparts (6-8).
Recent advances in translational research has enabled the classification of non-small cell lung cancer (NSCLC) into various molecular subsets defined by the so-called “driver mutations”, each with distinct clinicopathologic features as well as potential opportunities for targeted therapies (9). With the development of molecular targeted agents, currently about 15% of NSCLC patients benefit from the more personalized treatment protocols based on the genetic background of the tumor (10). For example, EGFR tyrosine kinase inhibitors (TKIs) such as Gefitinib and Erlotinib have been developed, and a subset of patients show significant therapeutic responses to this treatment, which was subsequently revealed to be associated with a mutation (11). In 2007, some patients who had ALK rearrangements were found to have similar remarkable responses to crizotinib, an ALK inhibitor (12). Other well-identified driver mutations in NSCLC include mutations in KRAS, HER2, BRAF, MEK, ERK and AKT1, as well as ALK and RET fusions, for which there are some novel oral TKIs in development (9,13,14). Noticeably, these oncogenes are frequently found in lung adenocarcinoma. On the other hand, mutations in tumor suppressor genes including TP53 and LKB1, which are also frequently seen in lung cancer, have been revealed to contribute to the pathogenesis of NSCLC as well (15-17). However, whether the youthful lung cancer has the exclusively distinct molecular features has not been well investigated.
Given the huge disease burden on public health due to the lung cancer in young patients, identifying its molecular and clinicopathologic features and thus making an appropriate treatment strategy is urgently necessary. In this study, we performed a comprehensive analysis of mutations in oncogenes and tumor suppressor genes in 36 Chinese young patients who were under 40 years old (including 40) from a single institution with surgically resected specimens. Moreover, we investigated the potential factors accounting for the early onset of lung cancer through comparing the molecular and clinicopathologic features with those of the older patients.
Materials and methods
Specimen collection
From October 2007 to November 2012, we consecutively collected lung tumor specimens resected with curative intent at Department of Thoracic Surgery in Fudan University Shanghai Cancer Center. Samples were snap-frozen in liquid nitrogen at the time of resection and stored at –80 °C until use. All cases were reviewed by pathologists for confirmation of tumor histology and tumor content. Subjects eligible in this study had to meet the following criteria: pathologically confirmed lung adenocarcinoma and sufficient tissue for comprehensive mutational analyses. This research was approved by the Institutional Review Board of Fudan University, Shanghai Cancer Center. Written informed consent was obtained from all patients.
Mutation analyses
RNA was extracted as per standard protocol after frozen tumor specimens were dissected into TRIzol (Invitrogen). Total RNA samples were reverse transcribed into single-stranded cDNA. EGFR (exons 18-22), KRAS (exons 2-3), HER2 (exons 18-21), BRAF (exons 11-15), AKT1 (exon 2), TP53 (exons 1-11) and LKB1 (exons 1-10) were amplified by reverse transcriptase polymerase chain reaction (rtPCR) using cDNA and directly sequenced. We examined the ALK and RET fusions using the rtPCR plus quantitative real-time PCR strategy, with validation using immunohistochemistry and fluorescent in situ hybridization (FISH) assays, which have been recently described (18,19). All PCR products were directly sequenced in forward and reverse directions. All mutations were verified by analysis of an independent PCR isolate.
Clinicopathologic variables
Clinicopathologic variables collected for analyses included gender, age at diagnosis, pathologic tumor-node-metastasis (pTNM) stage, tumor differentiation, family history, smoking status and histologic subtypes of adenocarcinoma according to the new IASLC/ATS/ERS multidisciplinary classification of lung adenocarcinoma (20). pTNM stages were evaluated in accordance with the seventh edition of the lung cancer staging classification system (21). Patients under 40 years old (including 40) were defined as the younger group and patients above 40 years old were defined as the older group.
Follow-up
The follow-up period was 4 months after surgery. The enhanced chest computed tomography (CT) scan and abdominal ultrasonography were performed every 4 months, while brain magnetic resonance imaging (MRI) and bone scanning were required every 6-8 months. If tumor recurrence or metastasis was suspected, the pathological evaluation was conducted if possible. The follow-up methods included the outpatient clinic registration and telephone contact.
Statistical analysis
The Pearson’s chi-squared test and Fisher’s exact test were used to analyze the mutational status and clinicopathologic features between the two groups. Overall survival (OS) was measured from the date of operation until the date of death from lung cancer or the date last seen alive. Those who died from other causes were censored at the date of death. The survival curves of OS were estimated by the Kaplan-Meier method. Differences in survival between the two groups were assessed by the log-rank test. All the statistical analyses were conducted in the SPSS 16.0 for Windows (SPSS Inc., Chicago, IL, USA). P values were two tailed for all the tests. P<0.05 was considered statistically significant.
Results
From October 2007 to November 2012, we consecutively collected a total of 2,676 resected lung cancer specimens. Corresponding clinical and pathological materials were procured as a database. According to the criteria, 36 lung adenocarinoma cases were enrolled in the younger group. Besides, 87 adenocarcinoma cases in which patients were older than 40 years old were consecutively collected as the older group during January 2008 to June 2010.
Clinicopathologic characteristics in the younger group and comparison with the older counterparts
In the younger group, there were 15 male and 21 female patients. Seven smokers and 29 non-smokers were identified. The mean age was 34.53±4.63 years old. Three patients had a family history of lung cancer and 7 patients had a family history of other malignancies. Surgical resections including 27 lobectomies, 8 wedge resections and 1 pneumonectomy were carried out for the younger patients. Final pathological results showed 3 adenocarcinomas in situ (AIS), 3 minimal invasive adenocarcinomas (MIA) and 30 invasive adenocarcinomas (invasive AD). And 18 IAs, 3 IBs, 1 IIA, 1 IIB, 12 IIAs and 1 IV were finally identified. When compared with these clinicopathologic characteristics in the older group, more early adenocarcinomas (P=0.033), more wedge resections (P<0.001) and fewer smokers (P=0.004) were seen (Table 1).
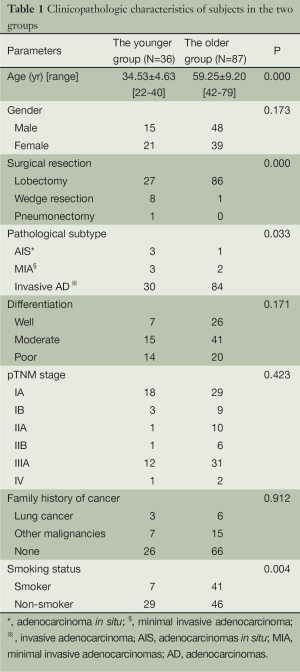
Full table
Molecular characteristics in the younger group and comparison with those in the older group
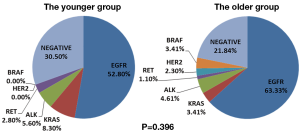
Nineteen EGFR mutations (52.8%), 3 KRAS mutations (8.3%), 2 EML4-ALK fusions (5.6%) and 1 KIF5b-RET fusion (2.8%) were identified in the younger group, while 55 EGFR mutations (63.2%), 3 KRAS mutations (3.4%), 4 EML4-ALK fusion (4.6%), 1 CCDC6-RET fusion (1.1%), 2 HER2 mutation (2.3%) and 3 BRAF mutations (3.4%) were found in the older group. The difference of oncogenic mutations between the two groups was statistically insignificant (P=0.396) (Figure 1). Among the 19 EGFR mutations in the younger group, there were 14 exon 19 deletions, 1 exon 20 insertions and 4 exon 21 missense mutations. The difference of EGFR mutation between the two groups was also insignificant (P=0.133) (Table 2).
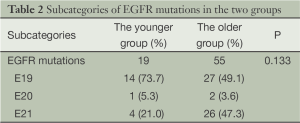
Full table
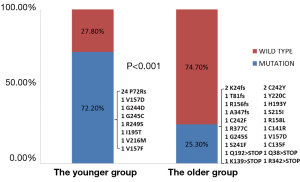
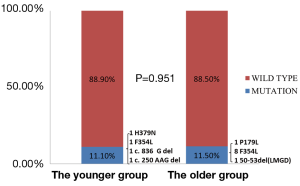
In addition, we evaluated mutations in tumor suppressor genes between the two groups. We found 26 TP53 mutations (72.2%) and 4 LKB1 mutations (11.1%) in young patients. When compared with these old patients, young patients showed a higher prevalence of TP53 mutations (P<0.001) and a comparable prevalence of LKB1 mutations (P=0.951) (Figures 2 and 3).
OS between the two groups
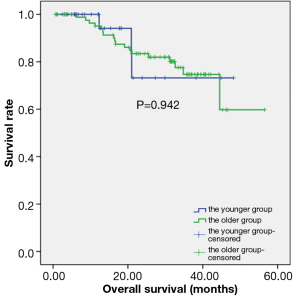
In the younger group, the median follow-up time was 14.15±11.77 months (range, 1-48.2 months), and the mean survival time was 40.39±3.94 months (95% CI: 32.66-48.11). In the older group, the median follow-up time was 31.20±1.21 months (range, 0.60-56.50 months), and the mean survival time was 45.57±2.44 months (95% CI: 40.79-50.35). There was no difference in the OS time between the two groups (P=0.942) (Figure 4).
Discussion
Lung cancer in young patients always attracts more attentions from the public because lung cancer is still the leading cause of cancer-related deaths worldwide (1) and young people are the backbone of the society. Though lung cancer usually affects patients in their 60s and 70s (4), age at diagnosis of lung cancer patients is keeping decreasing these years. Many studies have suggested that lung cancer in the young had its distinct clinicopathologic characteristics with different gender distribution, stage at diagnosis, pathological features and prognosis (4,5). However, the reported data are frequently discordant (6,7). Moreover, the molecular pathologic features of the youthful lung cancer have not been well investigated till now (22,23). In this study, we found that the youthful lung cancer had more early adenocarcinomas and fewer smoker patients (Table 1). And younger patients possessed the similar profiles of major oncogenic mutations in comparison with the older patients (Figure 1). Moreover, younger patients had a higher prevalence of TP53 mutations (72.2%) and a comparable prevalence of LKB1 mutations (11.1%) (Figures 2 and 3).
In the younger group, clinicopathologic features including gender distribution, differentiation of cancer cells, pTNM staging and family history of cancer were comparable with those in the older group, except that more AIS and MIA were seen and thus more wedge resections were performed, which were different from previous reports that younger patients always presented the more advanced stage at diagnosis (3,4,6,7). The discrepancies could be possibly explained that all patients in this study were candidates for surgical resections, and patients with advanced stage were not included. Moreover, the squamous cell carcinoma, which is always associated with tobacco use, was not enrolled because the prevalence of squamous cell carcinoma is low in the young and its molecular characteristics are still unclear.
Regarding the oncogenic characteristics, EGFR mutations were both predominant in the two groups (52.8% and 63.2%, respectively), followed by KRAS mutations, ALK and RET fusions. As a result, most young patients could benefit from the targeted therapies if indicated. On the other hand, in regard to mutations in tumor suppressor genes, the prevalence of TP53 mutations was 72.2% in the young patients, which is much higher than the rate in the older group. And it was also higher than the previous reports (24,25). Particularly, among the 26 TP53 point mutations, there were 24 P72R polymorphisms. And 5 P72Rs respectively coexisted with the missense mutations in TP53 (1 G244D, 1 G245C, 1 V157F, 1 I195T and 1 V216M, respectively). The P72R polymorphism has been associated with earlier age at first diagnosis of cancer, especially in germline TP53 mutation carriers, partly due to its modifier effect (15,26). Additionally, it has been suggested that familial susceptibility and Mendelian inheritance may produce the early onset of lung cancer (27-29). In this case, it may explain the early onset of the lung cancer in young patients. However, this needs further evaluations. Moreover, two deletions (c.836 G del; c.250 AAG del) and two point mutations (H379N; F354L) were found in LKB1 mutations. The prevalence of LKB1 mutations was comparable between the two groups, which indicated that LKB1 mutations were not particularly common in the youthful lung cancer.
Currently, there is no consensus about the specific cutoff age defining the youthful lung cancer, which could be resulted from different results on clinicopathologic patterns and prognosis of previously reported studies. Forty, forty-five and fifty are the three most common ages which are used to separate the young patients from the old ones (4-8). In this analysis, we selected patients who were under 40 years old (including 40) as the younger group. This age cut-off seems to be more reasonable, since the median age of patients with newly diagnosed NSCLC at presentation is 71 years (4). Surely, this issue is open to question.
OS time in the younger group was similar to that in the older group, although several studies suggested that younger patients with lung cancer had a better outcome than their older counterparts (4,6,7). In this study, all patients were candidates for surgical resection and the distribution of the pathological TNM stages was comparable between the two groups. Accordingly, younger patients with lung cancer could achieve a better prognosis if early detected and properly treated.
There were only two studies evaluating the molecular characteristics of lung adenocarcinoma in young patients aged 40 or younger before (22,23). In 2012, Nagashima and his colleagues analyzed EGFR, KRAS mutations and EML4-ALK fusion in twelve patients. They used direct sequencing for detecting EGFR mutation and performed FISH assay for detection of EML4-ALK fusion, however, their sample size was too small (22). Moreover, Kim and his colleagues also investigated EGFR mutation and ALK positivity in 31 young patients and 261 older patients with age >50 years last year. They did not find a statistical difference of the rate of EGFR mutation and ALK positivity between the two groups. However, they used the immunohistochemistry (IHC) assay for detection of EGFR mutation and ALK rearrangement in some small biopsy samples, which made their results less powerful (23). Furthermore, neither of them detected mutations in tumor suppressor genes. In this study, we firstly applied the standard methods for mutational detection in the surgically resected specimens and did the comprehensive analysis not only for major known oncogenic mutations, but also for mutations in tumor suppressor genes. We found a high prevalence of TP53 mutations and a comparable frequency of LKB1 mutations.
Though this study highlighted some impressive findings, several limitations in this study were worth noting. First, the sample size in this study was not enough big. If more young patients were available, our results would be more attractive. Moreover, patients with advanced disease were not enrolled since they could not provide enough tissues for comprehensive mutation evaluations. Thus the selection bias might influence our results. Furthermore, if the follow-up period was enough long, the survival analysis would be more powerful. However, most would agree that this study has really provided a brand new idea for the molecular and clinicopathologic features in the youthful lung cancer.
Conclusions
In conclusion, the youthful lung cancer unequivocally presented the distinct clinicopathologic characteristics including more early adenocarcinomas and fewer smoker patients. It showed the similar oncogenic characteristics compared with the older counterpart. Additionally, the prevalence of TP53 mutations was higher in the young patients, which may provide some insights on further investigations in the youthful lung cancer.
Acknowledgements
Funding: Project supported by Hospital Fund of Shanghai Cancer Center, Fudan University (Grant No. YJ201207) and the National Science Foundation for Young Scholars of China (Grant No.81302008).
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Levi F, Bosetti C, Fernandez E, et al. Trends in lung cancer among young European women: the rising epidemic in France and Spain. Int J Cancer 2007;121:462-5. [PubMed]
- Green LS, Fortoul TI, Ponciano G, et al. Bronchogenic cancer in patients under 40 years old. The experience of a Latin American country. Chest 1993;104:1477-81. [PubMed]
- Subramanian J, Morgensztern D, Goodgame B, et al. Distinctive characteristics of non-small cell lung cancer (NSCLC) in the young: a surveillance, epidemiology, and end results (SEER) analysis. J Thorac Oncol 2010;5:23-8. [PubMed]
- Antkowiak JG, Regal AM, Takita H. Bronchogenic carcinoma in patients under age 40. Ann Thorac Surg 1989;47:391-3. [PubMed]
- Radzikowska E, Roszkowski K, Głaz P. Lung cancer in patients under 50 years old. Lung Cancer 2001;33:203-11. [PubMed]
- Ramalingam S, Pawlish K, Gadgeel S, et al. Lung cancer in young patients: analysis of a Surveillance, Epidemiology, and End Results database. J Clin Oncol 1998;16:651-7. [PubMed]
- Sekine I, Nishiwaki Y, Yokose T, et al. Young lung cancer patients in Japan: different characteristics between the sexes. Ann Thorac Surg 1999;67:1451-5. [PubMed]
- Heist RS, Engelman JA. SnapShot: non-small cell lung cancer. Cancer Cell 2012;21:448.e2. [PubMed]
- Cufer T, Ovcaricek T, O’Brien ME. Systemic therapy of advanced non-small cell lung cancer: major-developments of the last 5-years. Eur J Cancer 2013;49:1216-25. [PubMed]
- Kobayashi K, Hagiwara K. Epidermal growth factor receptor (EGFR) mutation and personalized therapy in advanced nonsmall cell lung cancer (NSCLC). Target Oncol 2013;8:27-33. [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [PubMed]
- Goldman JW, Garon EB. Targeting MEK for the treatment of non-small-cell lung cancer. J Thorac Oncol 2012;7:S377-8. [PubMed]
- Guo Y, Du J, Kwiatkowski DJ. Molecular dissection of AKT activation in lung cancer cell lines. Mol Cancer Res 2013;11:282-93. [PubMed]
- Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol 2010;2:a001008. [PubMed]
- Ji H, Ramsey MR, Hayes DN, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature 2007;448:807-10. [PubMed]
- Gao Y, Xiao Q, Ma H, et al. LKB1 inhibits lung cancer progression through lysyl oxidase and extracellular matrix remodeling. Proc Natl Acad Sci U S A 2010;107:18892-7. [PubMed]
- Wang R, Pan Y, Li C, et al. The use of quantitative real-time reverse transcriptase PCR for 5' and 3' portions of ALK transcripts to detect ALK rearrangements in lung cancers. Clin Cancer Res 2012;18:4725-32. [PubMed]
- Wang R, Hu H, Pan Y, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol 2012;30:4352-9. [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [PubMed]
- Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest 2009;136:260-71. [PubMed]
- Nagashima O, Ohashi R, Yoshioka Y, et al. High prevalence of gene abnormalities in young patients with lung cancer. J Thorac Dis 2013;5:27-30. [PubMed]
- Kim L, Kim KH, Yoon YH, et al. Clinicopathologic and molecular characteristics of lung adenocarcinoma arising in young patients. J Korean Med Sci 2012;27:1027-36. [PubMed]
- Skaug V, Ryberg D, Kure EH, et al. p53 mutations in defined structural and functional domains are related to poor clinical outcome in non-small cell lung cancer patients. Clin Cancer Res 2000;6:1031-7. [PubMed]
- Ahrendt SA, Chow JT, Yang SC, et al. Alcohol consumption and cigarette smoking increase the frequency of p53 mutations in non-small cell lung cancer. Cancer Res 2000;60:3155-9. [PubMed]
- Bougeard G, Baert-Desurmont S, Tournier I, et al. Impact of the MDM2 SNP309 and p53 Arg72Pro polymorphism on age of tumour onset in Li-Fraumeni syndrome. J Med Genet 2006;43:531-3. [PubMed]
- Sellers TA, Bailey-Wilson JE, Elston RC, et al. Evidence for mendelian inheritance in the pathogenesis of lung cancer. J Natl Cancer Inst 1990;82:1272-9. [PubMed]
- Coté ML, Kardia SL, Wenzlaff AS, et al. Risk of lung cancer among white and black relatives of individuals with early-onset lung cancer. JAMA 2005;293:3036-42. [PubMed]
- Kharazmi E, Fallah M, Sundquist K, et al. Familial risk of early and late onset cancer: nationwide prospective cohort study. BMJ 2012;345:e8076. [PubMed]