
Digital health for COPD care: the current state of play
Introduction
Chronic obstructive pulmonary disease (COPD) is a severe chronic disease, which affects over 170 million people and accounts for over 3 million deaths each year globally (1). People with COPD often experience persistent respiratory symptoms such as dyspnoea (shortness of breath). They are also vulnerable to episodic COPD exacerbations (an acute worsening of respiratory symptoms requiring a change in treatment), triggered by various factors such as air pollution and infections (2). Uncontrolled COPD exacerbations often cause an irreversible loss in lung function (3) and/or complications requiring medical treatment in a hospital.
Because of the severity of symptoms, the care for patients with COPD needs to be extended from the in-patient care for acute events at the hospital to the outpatient care for long-term ongoing self-management of COPD in the community (4,5). Accordingly, extensive clinical intervention and support have been essential, such as regularly reviewing patients’ medical/health conditions, tailoring optimal treatments, promoting and supporting self-management, and sharing decisions or care plans for collaborative care. Delivery of such extensive COPD care is practically time-consuming and resource-intensive, especially under traditional care settings relying on paper-based records and face-to-face visits. Thus, there is a need for innovative care models for improving the efficiency and effectiveness of COPD care.
The use of information and communications technology (ICT) to deliver medical/clinical interventions remotely is one of the promising care solutions. Its initial endeavours aimed to improve access to care for people living in remote and rural areas by bridging the geographical barriers. The use of ICT for the provision of healthcare is referred to as telemedicine (6), often interchangeable with telehealth (7). Three main types of telemedicine have been described: synchronous (e.g., real-time video conferencing or telephone call), asynchronous (e.g., remote consultation using email, smartphone messages, and notifications), and remote monitoring (e.g., recording and communicating COPD symptoms to healthcare providers). With the increasing popularity of smartphones, it has become possible to provide some parts of COPD care through smartphone applications (apps). For example, numerous smartphone applications and personal healthcare devices have already been provided for patients to measure and/or record their health conditions such as COPD symptoms, physical activities and vital signs at home (8,9). The use of mobile devices for the provision of healthcare is called Mobile Health or mHealth (10), a subset of telemedicine.
Digital health is an overarching and relatively new term, which is defined as “the use of digital technologies for health” (11). Accordingly, digital health broadly include telemedicine applications, digital healthcare systems, electronic health records, and other applications using digital technologies for health. This overarching term hence encompasses electronic health (eHealth), telehealth, and mHealth. The terminology of this field is ambiguous and different terms are being used interchangeably (12). Through digital health applications, care providers in both hospital and primary care settings are able to easily share and review patients' data and, accordingly, provide optimal care plans and a personalized intervention. In addition, numerous analysis tools and parallel computing systems have also been made available to analyse the big data from the patients and care processes for improving care intervention and outcomes (13). Therefore, digital health transformation has been recognized as a promising driving force to empower individual patients, reduce health inequalities, and deliver innovative high-quality health care (11).
The aim of this review is to highlight the evidence base of digital health approaches for COPD care. We accordingly reviewed some typical studies and outcomes along the pathway from (I) ongoing self-management of stable COPD at home, (II) in-hospital care for acute illness and conditions under medical treatment, and (III) short-term post-discharge care programs for recovery through clinical follow-up, education and training, and (IV) hospital-at-home programs for moderate acute conditions and early discharge (Figure 1). We also reviewed several leading studies on impacts of environmental factors and public health surveillance, in which emerging environmental sensors, satellite images, wearable devices, and social media were used. We finally discussed the gaps and opportunities to improve COPD care outcomes for future studies.
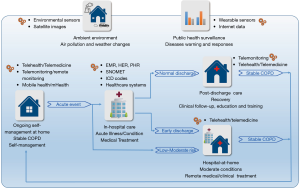
Ongoing self-management for stable COPD
People with COPD need to effectively manage their health condition on a daily basis, such as controlling COPD symptoms, modifying risk factors, and engaging with a multidisciplinary care team for timely interventions (4,5). This is essential to improve health-related quality of life, and reduce hospitalizations (14) and mortality (5,15). However, many patients are unable to comply with management requirements (16), because of limited health literacy, difficulties of using/accessing resources, and insufficient clinical support (16,17).
To improve the self-management of COPD, various digital health approaches such as mHealth (18,19), telemonitoring (20), and telemedicine (or telehealth) have been studied. These solutions enable care providers to remotely assess patients and provide intervention. Patients can also use various personal healthcare devices and Internet applications to access health information, monitor their conditions, and engage with the care team (18,19,21).
A recent review evaluated the incidences of acute COPD exacerbation in mHealth-enabled care programs in six randomized controlled trials (RCTs). It demonstrated that, compared with controls, participants in mHealth-enabled programs showed a reduction of 80% in acute exacerbations (3 RCTs, odds ratio of 0.20, P=0.005) (22). However, existing studies are limited to small sample sizes (n<100), with the issues of inaccurate alerts (23) and false positive events (missing exacerbation events in the monitoring processes) (24).
A large cluster randomised trial, named the Whole System Demonstrator, evaluated a telehealth-enabled program in people with diabetes (23.7%), COPD (48.3%), or heart failure (28.0%) (telehealth, n=1,584 vs. usual care, n=1,570) for 12 months (25). The telehealth intervention involved education, remote monitoring of patients’ symptoms and physiological measures, and clinical assessments for diagnosis and healthcare management by specialist nurses and community matrons. Compared with usual care, the telehealth group was found to significantly reduce hospital admissions by 18% (odds ratio 0.82, P=0.017) and mortality by 46% (odds ratio 0.54, P<0.001). Inconsistently, another relatively large RCT evaluated telemonitoring in patients with COPD, but did not find significant effects on reducing hospitalizations (telemonitoring, n=128, 1.2 admissions per person vs. controls, n=128, 1.1; P=0.59) and mortality (16 deaths vs. 21 deaths; adjusted odds ratio 0.66; 95% confidence interval or 95% CI: 0.29 to 1.48) (26). A potential reason for the non-significant effects was that the telemonitoring study used existing care services to support the telemonitoring intervention, whereas the telehealth intervention in the Whole System Demonstrator was supported by specialist care services and well-arranged general practices (26).
Several reviews demonstrated positive effects on reducing hospitalizations. Two telemonitoring reviews for COPD found that telemonitoring intervention significantly (P<0.05) reduced hospitalizations by over 20%, with reported risk ratios (RRs) of 0.72 (9 RCTs, 95% CI: 0.53 to 0.98, P<0.05) (27) and 0.78 (7 RCTs, 95% CI: 0.58–0.88) (28). A narrative telemonitoring review also showed risk reductions between 10% and 40% in 6 out of 8 RCTs (29). An mHealth review showed a reduction in hospital admissions, but the reduction was non-significant (6 RCTs, RR =0.73, 95% CI: 0.52 to 1.04) (30). Small sample sizes, moderate qualities, and heterogeneity were frequently reported by reviews (27,28,30), underscoring a need to further confirm hospitalization outcomes in future studies.
Clinical evidence on mortality from reviews remains limited and inconsistent. One telehealth review for COPD showed a lower mortality rate in telehealth intervention than usual care, but the effect was non-significant (2 RCTs, RR =0.96, 95% CI: 0.63–1.47) (28). Inconsistently, two other telehealth reviews for COPD found a higher mortality rate in telehealth programs than control with RR =1.05 from 3 RCTs (31) and RR =1.21 form 3 RCTs (32), although the findings were non-significant. Similarly, a higher mortality rate was also reported in three telemonitoring reviews for COPD, with RR =1.43 (4 RCTs, 95% CI: 0.40–5.03) (27), RR =1.36 (5 RCTs; 95% CI: 0.77–2.41) (33), and an increase in mortality (about 18% but non-significant) in two out of four RCTs (29).
In-hospital care
It is evident that, among digital health applications, electronic medical record (EMR) represents the greatest potential for improving healthcare quality. Using the EMR, care providers can efficiently share and access entire medical records of each patient for conducting urgent medical treatments or making optimal care plans. Besides care providers, patients can also easily review their records. This is essential for them to effectively adhere to the care plan and actively engage with a collaborative care team for optimal treatment (34).
Clinical evidence of EMR effects on in-hospital care for COPD remains limited. However, for general in-hospital care, the use of EMR has been shown to be beneficial. For example, studies have demonstrated potential advantages of using EMR in hospital care for efficient documentation, review, prescription, referral, and care management with reminders and messaging (35,36). The use of inpatient EMR has been shown to reduce duplicating pathology tests by 18% per week (37), length of stay (0.11 day) and 30-day mortality (0.182%) (38). An overview of clinical and organisational impact of digital technology in hospital practice demonstrated moderate-quality evidence for EMR on reducing hospitalisations and length of stay (39). The overview also showed an improvement in organisational efficiency, accuracy of information, documentation and turnaround times, although the evidence was of low-quality (39).
With regard to adoption of electronic records in healthcare services for improving savings, costs, healthcare quality and safety, a study demonstrated that the evidence is not robust enough to make strong predictions, and the existing findings only described as “potential” (40). This was mainly attributed to methodological shortcomings of the existing evidence; and the authors reported that the studies did not include many other effects of EMR such as transaction savings, reductions in malpractice costs, and research and public health savings (41).
Barriers to adoption of EMR include high costs, lack of certification and standardization, concerns about privacy, and a disconnection between who pays for EMR systems and who benefits from them. Furthermore, the issues of acquisition and implementation costs, slow and uncertain financial payoffs, and disruptive effects on practices often impede the adoption of EMR directly (40).
Besides EMR applications, advanced data analysis and management tools have also been developed and used. For example, the terminology tool of Systematized Nomenclature of Medicine-Clinical Terms (SNOMED-CD) (42,43) has been applied to address inconsistencies in the documentation of medical procedures and interventions. International Classification of Diseases, 10th Revision (ICD-10) has also been applied for consistent classification of various conditions and treatments (44). These applications potentially improve analysis and interpretation of electronic records in clinical studies (43,44). Implementations of computerized decision support system in inpatient care have also been found with a potential to reduce mortality and life-threatening events (45). However, these digital health approaches have not been fully explored for improving COPD care. Therefore, research in these aspects are warranted in future COPD studies.
Short-term post-discharge care
COPD is a leading cause of hospital re-admissions, with reported 30-day re-admission rates of 20% (46). The reasons for re-admission are complex, often associated with breathing difficulty (47), respiratory deterioration, and lack of home care plan (46). Post discharge care has therefore been essential to assist patients in addressing health issues. This often involves clinical follow-up and examination, gas exchanges, and care planning in a multidisciplinary team approach (5). Studies have demonstrated comprehensive post-discharge care programs potential reduced early hospital readmissions in traditional care settings (48-50).
To date, the use of digital health for post-discharge care in COPD patients remains limited and inconsistent. Sorknaes et al. evaluated a telemedicine-enabled post-discharge care program with 4-week follow up in a cohort study. In that study, participants in the intervention arm were provided with the telemedicine system within 24 hours after initial hospital discharge. The system consisted of a computer with a web camera, a microphone, and measurement equipment for assessing blood oxygen saturation and lung function. A nurse-led care team remotely assessed the patients and assisted them in preventing exacerbations and using the medication. The intervention arm (n=50) was then compared with control (usual care, n=50). The results demonstrated a significant reduction in 30-day re-admissions in the intervention arm (hazard ratio 0.25, 95% CI: 0.09–0.69) (51). Inconsistently, in another study, Kerenidi et al. evaluated a short-term telemonitoring program after hospital discharge for COPD exacerbation in an RCT. While comparing the telemonitoring program (n=83) with usual care (n=32), they could not find significant differences in hospital re-admissions between the two groups (52).
Pulmonary rehabilitation is an evidence-based clinical program to help COPD patients manage their conditions after discharging from hospital. It normally comprises education and training sessions for 6–8 weeks. Conventional pulmonary rehabilitation is effective, but often underutilized (53) because of unavailability, time constraints, limited knowledge, and difficulties for travelling (54). Telemonitoring has been used to address this issue. For example, several feasibility studies have demonstrated that technology applications are feasible and acceptable for home-based pulmonary rehabilitation programs (55-57). A RCT was conducted to evaluate telehealth support via Skype for teaching pursed lips breathing and coordination of breathing during physical activity. The study demonstrated that the telehealth intervention group improved the patient adherence to the program and resulted in a lower level of dyspnoea intensity (58). In a non-inferiority study, a telehealth-enabled pulmonary rehabilitation program resulted in improvements in quality of life and 12-min walk distance, similar to the centre-based pulmonary rehabilitation (59). Consistently, a systematic review evaluated telemonitoring-enabled cardiac or pulmonary rehabilitation programs (n=8). It demonstrated that technology-enabled rehabilitation programs improved the level of exercise and the improvements were similar to those in traditional centre-based programs (60). Despite these positive findings, the clinical evidence remains limited (60). Therefore, more clinical trials are needed to confirm the effectiveness in future studies.
Hospital-at-home
Hospital-at-home (or Home Hospitalization) is “a service that provides active treatment by healthcare professionals in the patient’s home for a condition that otherwise would require acute hospital inpatient care” (61). Hospital-at-home programs offer an alternative for patients requiring treatment at the hospital or emergency department. It also allows care providers to discharge patients at an early stage, and continue to treat the patients at home. Clinical guidelines for management of exacerbation at home have been provided (62).
A telehealth program to support hospital-at-home for COPD exacerbation was evaluated in an RCT. The study demonstrated the feasibility of using the program to treat COPD exacerbation, but did not find significant effects on reducing hospitalizations and mortality likely because of the limited sample size (n=57) (63). Clinical evidence from recent reviews (64,65) remains limited to conclude any healthcare outcomes.
Public health surveillance using social media and sensors
Public health surveillance is traditionally based on collecting and analysing health-related data from general practices (GPs), clinic centres, hospitals and government departments for evaluating disease outbreaks and improving diseases early warning and responses. With advances in digital transformation, various new data from populations or cohorts of millions of people have been studied to improve public health surveillance.
Recently, health records (66), genome sequencing, behaviour patterns (67), measures from environmental sensors, social media (68), internet usage (69,70) and personal health data from wearable and personal healthcare devices have been used to improve communicable and non-communicable diseases surveillance (71). These studies help understand disease causation and transmission patterns from different aspects and, then, support development of optimal care strategies for improving healthcare outcomes (72).
Social media such as online forums and Twitter provide convenient open platforms for conversations around health related topics. The conversations often reflect actual patients’ opinions, issues, or care needs in everyday life, which are usually unavailable in clinical assessments and traditional healthcare registries. For example, Cook and colleagues analysed the posts (n=849) from 695 unique records (82% from patients with COPD and 18% from caregivers). They then demonstrated the predominant topics of cough (27%), mucus production (25%), and shortness of breath (21%), indicating the need for clinical support to practically assist the patients in alleviating these symptoms in COPD care (73).
Ambient environment
Recently, environmental sensors and satellite images have been used to analyse the environmental factors in associations with COPD. Through these applications, large cohort studies have demonstrated that concentrations of air pollutants such as particulate matter (PM) with aerodynamic diameter <2.5 µm (PM2.5) were associated with declined lung functions (74,75), the risk for COPD and chronic bronchitis (76), and a potential increase in the prevalence and incidence of COPD (77).
In another large study, Qiu and colleagues extracted a total of 54,966 COPD hospital admissions (male: female ratio =1.8:1) from 124 hospitals in the urban areas of Chengdu, China (78). They then analysed the admissions in association with ambient air pollutants including PM10, PM2.5, nitrogen dioxide (NO2), sulfur dioxide (SO2), carbon monoxide (CO) and ozone (O3). The analysis results demonstrated that hospital admissions for COPD were associated with concentrations of ambient air pollutants (PM2.5, PM10 and SO2) and cold whether (low ambient temperature) (78). Consistently, a recent systematic review with meta-analysis evaluated 1,115,000 COPD-related acute events (950,000 hospital admissions, 80,000 emergency department visits, and 130,000 deaths) from 37 studies. The evaluation results demonstrated that ambient outdoor concentrations of PM2.5, NO2, and SO2 were significantly associated with hospital admissions, emergency department visits, and deaths (79).
World Health Organization (WHO) has called for global efforts to assess and address of the burden of various diseases including COPD from environmental risks, through policies, interventions, and technologies. Because of the recent recognitions of environmental risk factors to COPD, numerous personal devices have recently been developed to help patients with COPD or other respiratory diseases monitor ambient air pollution and prevent air-pollution-related COPD symptoms and hospitalizations (80).
Discussions and future directions
This study reviewed various digital transformation approaches for COPD care in a broad scope. It demonstrated that telemedicine including the subsets of telemonitoring and mHealth was predominantly applied to improve the self-management of COPD and post-discharge care. These applications showed a potential to reduce hospitalizations, and indicated a need for rigorously improving and evaluating effects on mortality in future studies. In hospital care, various digital technologies such as EMR, SNOMET, ICD codes and computerized decision support systems have been implemented, but practical use of the technologies for COPD remains limited. Air pollution in ambient environment has been found to increase the risk for hospitalization and mortality, but use of the findings to prevent hospitalization and mortality remains absent and, hence, need to be further explored. Similarly, the clinical evidence of using digital technologies in public health surveillance and hospital-at-home remains insufficient to draw any firm conclusions.
Potential issues have also been found through this review, including an increased musculoskeletal adverse events in an Internet-application-based COPD care program for exercise intervention (81), and a higher risk for mortality (despite non-significance) in telemonitoring enabled care programs presented. Frequent remote assessments and consultations have also been found to dramatically increase care burdens to the care providers (24). These issues practically deter the digital health transformation and need to be carefully addressed and rigorously evaluated in future studies. Unlike typical clinical and pharmaceutical trials, the evaluation of digital health interventions is not simple. A comprehensive evaluation and assessment of these interventions should consider the maturity level of the digital system understudy. A simple model of evaluation for digital health and eHealth suggests a suite of research studies in five stages (Concept development, Service design, Pre-implementation, Implementation, and Post-implementation) to be designed and carried out to produce robust evidence on the effects and cost of such interventions (82).
Effective detection of acute COPD exacerbation is a major clinical objective in COPD care (5), but remains difficult to achieve. Clinical diagnosis of acute exacerbation mainly relies on subjective assessment of COPD symptoms. So far, no objective measures through digital transformation approaches have been found to reliably predict acute exacerbations. Therefore, research on innovative monitoring devices and predictive models is warranted to improve telemonitoring interventions in the future.
Both significant and non-significant effects have been reported in telemedicine-based study for the self-management of COPD. The inconsistent outcomes could be caused by heterogeneous designs of telemonitoring intervention. Therefore, it is essential to identify underpinning care processes contributing to care improvements. In addition, patients’ health conditions and therapeutic responses normally differ remarkably. Therefore, identifying ideal candidates and/or effective personalized intervention strategies would be essential to achieve reliable clinical outcomes in future studies (83).
Many studies have developed and evaluated digital health solutions for improving the self-management of COPD. However, the implementation of these solutions was not always successful, because of technical difficulties, low patient compliance, and lack of personalisation. To address this issue, an iterative approach with continuous improvement is essential. In addition, acknowledging and addressing users’ requests often improve uptake and adherence. Digital health systems that have been designed with patient-centred approach and allowed personalisation of the service often yield higher usability and compliance rate by both patients and healthcare professionals (84).
The complexity of the digital health for self-management of COPD has escalated with recent advancement in digital technology (e.g., smartphones and wearable devices) as well as computer processing power and capabilities. As a result, artificial intelligence (AI) is emerging and finding its way into health sector. Like many other disciplines, pulmonary medicine has been the topic of a number of research studies and experiments for AI applications. Most of the experiments with AI for obstructive lung diseases to date have been around diagnosis (using data from pulmonary function tests and computed tomography scanning), but the latest trend of research in this area has shown interest in assessing COPD symptoms, such as the analysis of lung sounds using deep learning techniques (85). Intelligent analysis of personalised data which are collected via smartphone sensors and wearable devices provides new opportunities for early prediction and prevention of COPD exacerbation.
Conclusions
Through digital transformation, new data, innovative applications and care models have been increasingly studied to improve COPD care. Potentials of using digital-technology-enabled care programs to reduce hospitalizations and mortality have been demonstrated in many studies, but the clinical evidence often is limited and/or inconsistent. Therefore, research for achieving reliable beneficial outcomes remains needed in future studies.
Acknowledgments
H Ding received funding from The Prince Charles Hospital Foundation. F Fatehi received funding from the Queensland Government through an Advance Queensland Research Fellowship. N Bashi is funded by the Australian e-Health Research Centre, Commonwealth Scientific and Industrial Research Organisation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med 2017;5:691-706. [Crossref] [PubMed]
- Viniol C, Vogelmeier CF. Exacerbations of COPD. Eur Respir Rev 2018;27:170103. [Crossref] [PubMed]
- Qureshi H, Sharafkhaneh A, Hanania NA. Chronic obstructive pulmonary disease exacerbations: latest evidence and clinical implications. Ther Adv Chronic Dis 2014;5:212-27. [Crossref] [PubMed]
- Singh D, Agusti A, Anzueto A, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: the GOLD science committee report 2019. Eur Respir J 2019;53:1900164. [Crossref] [PubMed]
- Yang IA, Brown JL, George J, et al. COPD-X Australian and New Zealand guidelines for the diagnosis and management of chronic obstructive pulmonary disease: 2017 update. Med J Aust 2017;207:436-42. [Crossref] [PubMed]
- Ryu S. Telemedicine: Opportunities and Developments in Member States: Report on the Second Global Survey on eHealth 2009 (Global Observatory for eHealth Series, Volume 2). Healthc Inform Res 2012;18:153-5. [Crossref]
- Tuckson RV, Edmunds M, Hodgkins ML. Telehealth. New England Journal of Medicine 2017;377:1585-92. [Crossref] [PubMed]
- Sobnath DD, Philip N, Kayyali R, et al. Features of a Mobile Support App for Patients With Chronic Obstructive Pulmonary Disease: Literature Review and Current Applications. JMIR Mhealth Uhealth 2017;5:e17. [Crossref] [PubMed]
- Aliverti A. Wearable technology: role in respiratory health and disease. Breathe (Sheff) 2017;13:e27-36. [Crossref] [PubMed]
- WHO. mHealth: new horizons for health through mobile technologies: second global survey on eHealth. Geneva: World Health Organization; 2011.
- World Health Organization. WHO guideline: recommendations on digital interventions for health system strengthening. Geneva: World Health Organization 2019.
- Fatehi F, Wootton R. Telemedicine, telehealth or e-health? A bibliometric analysis of the trends in the use of these terms. J Telemed Telecare 2012;18:460-4. [Crossref] [PubMed]
- Bhavnani SP, Parakh K, Atreja A, et al. 2017 Roadmap for Innovation-ACC Health Policy Statement on Healthcare Transformation in the Era of Digital Health, Big Data, and Precision Health: A Report of the American College of Cardiology Task Force on Health Policy Statements and Systems of Care. J Am Coll Cardiol 2017;70:2696-718. [Crossref] [PubMed]
- Zwerink M, Brusse-Keizer M, van der Valk PD, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014.CD002990. [PubMed]
- World Health Organization. COPD management. Available online: http://www.who.int/respiratory/copd/management/en/. Accessed 11 November 2017.
- Horie J, Murata S, Hayashi S, et al. Factors that delay COPD detection in the general elderly population. Respir Care 2011;56:1143-50. [Crossref] [PubMed]
- Sanduzzi A, Balbo P, Candoli P, et al. COPD: adherence to therapy. Multidiscip Respir Med 2014;9:60. [Crossref] [PubMed]
- Ding H, Karunanithi M, Kanagasingam Y, et al. A pilot study of a mobile-phone-based home monitoring system to assist in remote interventions in cases of acute exacerbation of COPD. J Telemed Telecare 2014;20:128-34. [Crossref] [PubMed]
- Ding H, Moodley Y, Kanagasingam Y, et al. A mobile-health system to manage chronic obstructive pulmonary disease patients at home. Conf Proc IEEE Eng Med Biol Soc 2012;2012:2178-81. [PubMed]
- Miłkowska-Dymanowska J, Bialas AJ, Obrebski W, et al. A pilot study of daily telemonitoring to predict acute exacerbation in chronic obstructive pulmonary disease. Int J Med Inform 2018;116:46-51. [Crossref] [PubMed]
- Nguyen HQ, Donesky-Cuenco D, Wolpin S, et al. Randomized controlled trial of an internet-based versus face-to-face dyspnea self-management program for patients with chronic obstructive pulmonary disease: pilot study. J Med Internet Res 2008;10:e9. [Crossref] [PubMed]
- Alwashmi M, Hawboldt J, Davis E, et al. The Effect of Smartphone Interventions on Patients With Chronic Obstructive Pulmonary Disease Exacerbations: A Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth 2016;4:e105. [Crossref] [PubMed]
- Gerdes M, Gallefoss F, Fensli RW. The EU project "United4Health": Results and experiences from automatic health status assessment in a Norwegian telemedicine trial system. J Telemed Telecare 2019;25:46-53. [Crossref] [PubMed]
- Ure J, Pinnock H, Hanley J, et al. Piloting tele-monitoring in COPD: a mixed methods exploration of issues in design and implementation. Prim Care Respir J 2012;21:57-64. [Crossref] [PubMed]
- Steventon A, Bardsley M, Billings J, et al. Effect of telehealth on use of secondary care and mortality: findings from the Whole System Demonstrator cluster randomised trial. BMJ 2012;344:e3874. [Crossref] [PubMed]
- Pinnock H, Hanley J, McCloughan L, et al. Effectiveness of telemonitoring integrated into existing clinical services on hospital admission for exacerbation of chronic obstructive pulmonary disease: researcher blind, multicentre, randomised controlled trial. BMJ 2013;347:f6070. [Crossref] [PubMed]
- Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract 2014;68:369-78. [Crossref] [PubMed]
- Yang F, Xiong ZF, Yang C, et al. Continuity of Care to Prevent Readmissions for Patients with Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Copd 2017;14:251-61. [Crossref] [PubMed]
- Pedone C, Lelli D. Systematic review of telemonitoring in COPD: an update. Pneumonol Alergol Pol 2015;83:476-84. [Crossref] [PubMed]
- Yang F, Wang Y, Yang C, et al. Mobile health applications in self-management of patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis of their efficacy. BMC Pulm Med 2018;18:147. [Crossref] [PubMed]
- McLean S, Nurmatov U, Liu JL, et al. Telehealthcare for chronic obstructive pulmonary disease: Cochrane Review and meta-analysis. Br J Gen Pract 2012;62:e739-49. [Crossref] [PubMed]
- Polisena J, Tran K, Cimon K, et al. Home telehealth for chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Telemed Telecare 2010;16:120-7. [Crossref] [PubMed]
- Kamei T, Yamamoto Y, Kajii F, et al. Systematic review and meta-analysis of studies involving telehome monitoring-based telenursing for patients with chronic obstructive pulmonary disease. Jpn J Nurs Sci 2013;10:180-92. [Crossref] [PubMed]
- Biggs JS, Willcocks A, Burger M, et al. Digital health benefits evaluation frameworks: building the evidence to support Australia's National Digital Health Strategy. Med J Aust 2019;210 Suppl 6:S9-11. [Crossref] [PubMed]
- Perri-Moore S, Kapsandoy S, Doyon K, et al. Automated alerts and reminders targeting patients: A review of the literature. Patient Educ Couns 2016;99:953-9. [Crossref] [PubMed]
- Penoyer DA, Cortelyou-Ward KH, Noblin AM, et al. Use of electronic health record documentation by healthcare workers in an acute care hospital system. J Healthc Manag 2014;59:130-44. [Crossref] [PubMed]
- Zlabek JA, Wickus JW, Mathiason MA. Early cost and safety benefits of an inpatient electronic health record. J Am Med Inform Assoc 2011;18:169-72. [Crossref] [PubMed]
- Lee J, Kuo Y-F, Goodwin JS. The effect of electronic medical record adoption on outcomes in US hospitals. BMC Health Serv Res 2013;13:39. [Crossref] [PubMed]
- Keasberry J, Scott IA, Sullivan C, et al. Going digital: a narrative overview of the clinical and organisational impacts of eHealth technologies in hospital practice. Aust Health Rev 2017;41:646-64. [Crossref] [PubMed]
- Miller RH, Sim I. Physicians’ Use of Electronic Medical Records: Barriers and Solutions. Health Aff (Millwood) 2004;23:116-26. [Crossref] [PubMed]
- Hillestad R, Bigelow J, Bower A, et al. Can Electronic Medical Record Systems Transform Health Care? Potential Health Benefits, Savings, And Costs. Health Aff (Millwood) 2005;24:1103-17. [Crossref] [PubMed]
- Agency ADH. SNOMED CT-AU. 2019. Available online: https://www.healthterminologies.gov.au/access.
- Hansen DP, Kemp ML, Mills SR, et al. Developing a national emergency department data reference set based on SNOMED CT. Med J Aust 2011;194:S8-10. [Crossref] [PubMed]
- Chabra S. International Classification of Diseases, 10th Revision, coding for prematurity: need for standardized nomenclature. Health Care Manag (Frederick) 2015;34:123-7. [Crossref] [PubMed]
- Varghese J, Kleine M, Gessner SI, et al. Effects of computerized decision support system implementations on patient outcomes in inpatient care: a systematic review. J Am Med Inform Assoc 2018;25:593-602. [Crossref] [PubMed]
- Shah T, Churpek MM, Coca Perraillon M, et al. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest 2015;147:1219-26. [Crossref] [PubMed]
- Rezaee ME, Ward CE, Nuanez B, et al. Examining 30-day COPD readmissions through the emergency department. Int J Chron Obstruct Pulmon Dis 2017;13:109-20. [Crossref] [PubMed]
- Alshabanat A, Otterstatter MC, Sin DD, et al. Impact of a COPD comprehensive case management program on hospital length of stay and readmission rates. Int J Chron Obstruct Pulmon Dis 2017;12:961-71. [Crossref] [PubMed]
- Russo AN, Sathiyamoorthy G, Lau C, et al. Impact of a Post-Discharge Integrated Disease Management Program on COPD Hospital Readmissions. Respir Care 2017;62:1396-402. [Crossref] [PubMed]
- Sharma G, Kuo YF, Freeman JL, et al. Outpatient follow-up visit and 30-day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med 2010;170:1664-70. [Crossref] [PubMed]
- Sorknaes AD, Madsen H, Hallas J, et al. Nurse tele-consultations with discharged COPD patients reduce early readmissions--an interventional study. Clin Respir J 2011;5:26-34. [Crossref] [PubMed]
- Kerenidi T, Stafyla E, Dafoulas G, et al. Short-term telemonitoring program after hospital discharge for COPD exacerbation: Greek pilot of the renewing health multicenter randomized trial. Eur Respir J 2015;46:OA3276.
- Sundh J, Lindgren H, Hasselgren M, et al. Pulmonary rehabilitation in COPD - available resources and utilization in Swedish primary and secondary care. Int J Chron Obstruct Pulmon Dis 2017;12:1695-704. [Crossref] [PubMed]
- Cox NS, Oliveira CC, Lahham A, et al. Pulmonary rehabilitation referral and participation are commonly influenced by environment, knowledge, and beliefs about consequences: a systematic review using the Theoretical Domains Framework. J Physiother 2017;63:84-93. [Crossref] [PubMed]
- Bonnevie T, Gravier FE, Elkins M, et al. People undertaking pulmonary rehabilitation are willing and able to provide accurate data via a remote pulse oximetry system: a multicentre observational study. J Physiother 2019;65:28-36. [Crossref] [PubMed]
- Seidman Z, McNamara R, Wootton S, et al. People attending pulmonary rehabilitation demonstrate a substantial engagement with technology and willingness to use telerehabilitation: a survey. J Physiother 2017;63:175-81. [Crossref] [PubMed]
- Paneroni M, Colombo F, Papalia A, et al. Is Telerehabilitation a Safe and Viable Option for Patients with COPD? A Feasibility Study. Copd 2015;12:217-25. [Crossref] [PubMed]
- Nield M, Hoo GW. Real-time telehealth for COPD self-management using Skype. Copd 2012;9:611-9. [Crossref] [PubMed]
- Stickland M, Jourdain T, Wong EYL, et al. Using Telehealth technology to deliver pulmonary rehabilitation in chronic obstructive pulmonary disease patients. Can Respir J 2011;18:216-20. [Crossref] [PubMed]
- Chan C, Yamabayashi C, Syed N, et al. Exercise Telemonitoring and Telerehabilitation Compared with Traditional Cardiac and Pulmonary Rehabilitation: A Systematic Review and Meta-Analysis. Physiother Can 2016;68:242-51. [Crossref] [PubMed]
- Gonçalves-Bradley DC, Iliffe S, Doll HA, et al. Early discharge hospital at home. Cochrane Database Syst Rev 2017;6:CD000356. [PubMed]
- British Thoracic Society Guideline Development Group. Intermediate care—Hospital-at-Home in chronic obstructive pulmonary disease: British Thoracic Society guideline. Thorax 2007;62:200-10. [Crossref] [PubMed]
- Jakobsen AS, Laursen LC, Rydahl-Hansen S, et al. Home-based telehealth hospitalization for exacerbation of chronic obstructive pulmonary disease: findings from "the virtual hospital" trial. Telemed J E Health 2015;21:364-73. [Crossref] [PubMed]
- Davies L, Wilkinson M, Bonner S, et al. "Hospital at home" versus hospital care in patients with exacerbations of chronic obstructive pulmonary disease: prospective randomised controlled trial. Bmj 2000;321:1265-8. [Crossref] [PubMed]
- Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev 2016;9:CD007491. [PubMed]
- Niyonsenga T, Coffee NT, Del Fante P, et al. Practical utility of general practice data capture and spatial analysis for understanding COPD and asthma. BMC Health Serv Res 2018;18:897. [Crossref] [PubMed]
- Strain T, Wijndaele K, Brage S. Physical Activity Surveillance Through Smartphone Apps and Wearable Trackers: Examining the UK Potential for Nationally Representative Sampling. JMIR Mhealth Uhealth 2019;7:e11898. [Crossref] [PubMed]
- Gittelman S, Lange V, Gotway Crawford CA, et al. A new source of data for public health surveillance: Facebook likes. J Med Internet Res 2015;17:e98. [Crossref] [PubMed]
- Milinovich GJ, Magalhaes RJ, Hu W. Role of big data in the early detection of Ebola and other emerging infectious diseases. Lancet Glob Health 2015;3:e20-1. [Crossref] [PubMed]
- Milinovich GJ, Williams GM, Clements AC, et al. Internet-based surveillance systems for monitoring emerging infectious diseases. Lancet Infect Dis 2014;14:160-8. [Crossref] [PubMed]
- Konty KJ, Bradshaw B, Ramirez E, et al. Influenza Surveillance Using Wearable Mobile Health Devices. Online J Public Health Inform 2019;11:e249. [Crossref]
- Khoury MJ, Iademarco MF, Riley WT. Precision Public Health for the Era of Precision Medicine. Am J Prev Med 2016;50:398-401. [Crossref] [PubMed]
- Cook NS, Kostikas K, Gruenberger JB, et al. Patients' perspectives on COPD: findings from a social media listening study. ERJ Open Res 2019. [Crossref] [PubMed]
- Adam M, Schikowski T, Carsin AE, et al. Adult lung function and long-term air pollution exposure. ESCAPE: a multicentre cohort study and meta-analysis. Eur Respir J 2015;45:38-50. [Crossref] [PubMed]
- Mudway IS, Dundas I, Wood HE, et al. Impact of London's low emission zone on air quality and children's respiratory health: a sequential annual cross-sectional study. Lancet Public Health 2019;4:e28-e40. [Crossref] [PubMed]
- Kurmi OP, Semple S, Simkhada P, et al. COPD and chronic bronchitis risk of indoor air pollution from solid fuel: a systematic review and meta-analysis. Thorax 2010;65:221-8. [Crossref] [PubMed]
- Schikowski T, Mills IC, Anderson HR, et al. Ambient air pollution: a cause of COPD? Eur Respir J 2014;43:250-63. [Crossref] [PubMed]
- Qiu H, Tan K, Long F, et al. The Burden of COPD Morbidity Attributable to the Interaction between Ambient Air Pollution and Temperature in Chengdu, China. Int J Environ Res Public Health 2018;15:15. [Crossref] [PubMed]
- DeVries R, Kriebel D, Sama S. Outdoor Air Pollution and COPD-Related Emergency Department Visits, Hospital Admissions, and Mortality: A Meta-Analysis. COPD 2017;14:113-21. [Crossref] [PubMed]
- Larkin A, Hystad P. Towards Personal Exposures: How Technology Is Changing Air Pollution and Health Research. Curr Environ Health Rep 2017;4:463-71. [Crossref] [PubMed]
- Moy ML, Collins RJ, Martinez CH, et al. An Internet-Mediated Pedometer-Based Program Improves Health-Related Quality-of-Life Domains and Daily Step Counts in COPD: A Randomized Controlled Trial. Chest 2015;148:128-37. [Crossref] [PubMed]
- Fatehi F, Smith AC, Maeder A, et al. How to formulate research questions and design studies for telehealth assessment and evaluation. J Telemed Telecare 2017;23:759-63. [Crossref] [PubMed]
- Vitacca M, Montini A, Comini L. How will telemedicine change clinical practice in chronic obstructive pulmonary disease? Ther Adv Respir Dis 2018;12:1753465818754778. [Crossref] [PubMed]
- Velardo C, Shah SA, Gibson O, et al. Digital health system for personalised COPD long-term management. BMC Med Inform Decis Mak 2017;17:19. [Crossref] [PubMed]
- Altan G, Kutlu Y, Allahverdi N. Deep Learning on Computerized Analysis of Chronic Obstructive Pulmonary Disease. IEEE J Biomed Health Inform 2019. [Epub ahead of print]. [Crossref] [PubMed]


