Prevalence and risk factors of aortic aneurysm in patients with chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of morbidity and mortality worldwide. The characteristic feature is progressive airflow limitation related to a chronic inflammatory response in the lung (1). Recently, epidemiological studies have demonstrated that COPD patients manifest an excess of chronic co-morbidities, mainly cardiovascular, which are well-known as important factors that determine prognosis and functional capabilities (2-7). The prevalence of coronary artery diseases and heart failure in patients with COPD has been reported to be associated with airflow obstruction and the extent of emphysema. Moreover, the percentage of cardiovascular-related mortality is up to twofold higher than in a matched population without COPD (2,3).
Aortic aneurysm (AA) is a common and potentially life-threatening condition. Advanced age and smoking are risk factors that connected with COPD as they are for other cardiovascular diseases. Some studies reveal that COPD is associated with the development of AA and appears to convey an added risk of rupture in patients with small aneurysms (8,9). Furthermore, in the 4-year Understanding Potential Long-Term Impacts on Function with Tiotropium (UPLIFT) trial, AA rupture was reported as one of the common causes of death as well as myocardial infarction and colorectal cancer. That is, the incidence of AA was twice or more as frequent as that of pulmonary embolism or prostate cancer (10). Together, these previous reports suggest that COPD patients, like those with coronary artery disease and heart failure, have a comparatively higher risk of AA than non-COPD smokers. To determine its prevalence and risk factors, we performed the cross-sectional, case-control study in a major Japanese medical institution.
Methods
Patient population and study design
We recruited participants from patients who had a prior diagnosis of COPD and regularly visited the Department of Respiratory Medicine at Kameda Medical Center (Chiba, Japan) between April 2011 and March 2012. The inclusion criteria were: aged >40 years, a smoking history of at least 20 pack-year, and a post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio <70% at inclusion. In this study, we also included patients whose AA was previously diagnosed, but excluded patients with a history of lung resection or other significant respiratory diseases. Then, finally, 231 patients were enrolled and underwent CT scanning of the chest, abdomen and pelvis, in addition to the regular COPD workup that including physical examination, pulmonary function testing, and chest radiographs.
The presence of AA was diagnosed by one radiologist and independently confirmed by two pulmonologists (KA and NK) on the basis of CT findings such as its size, length, location and shape, etc. Then, we designated 33 patients whose AA was documented either by our current CT scans (n=27) or former diagnoses (n=6) as “patients with AA (AA group)”, and classified the remaining 198 without AA of 231 patients initially enrolled into a non-AA group. We compared both groups’ backgrounds and CT data. The study protocol was approved by the ethics committee of Kameda Medical Center, and written informed consent was obtained from each participant.
Pulmonary function tests
Pulmonary function tests were performed according to American Thoracic Society standards, with a Chestac-65V (Chest MI Corp, Tokyo, Japan). FEV1 for each patient is expressed as a percentage of the predicted value (FEV1% predicted), which was calculated based on reference values from the Japanese population (11).
Analyses of CT images
All CT examinations were performed with commercially available CT scanners (X-force SH, Toshiba Medical, Tokyo, Japan), and non-enhanced CT scans were obtained with 5-mm section thickness. The severity of emphysema was visually assessed on CT images of the chest by two pulmonologists (KA and NK), and its extent, the percentage of lung destruction, was classified according to the Goddard scoring system (12): score 0, normal; score 1, ≤25% affected; score 2, <25% and ≤50% affected; score 3, 50% and ≤75% affected; and score 4, >75% affected. Six images were analyzed in three slices, which were obtained from 1 cm above the upper margin of the aortic arch, 1 cm below the carina, and 1 cm above the right diaphragm, and a total score of six images was calculated in each person.
Meanwhile, the aortic calcification index (ACI) was recently reported as a useful marker for evaluating abdominal aortic calcification and in association with the prevalence of cardiovascular disease (13-16). To assess ACI, we selected ten slices from each patient at 1 cm intervals from the common iliac bifurcation upward and calculated the mean percentage of the calcification area (divided into 12 sectors).
Although this index is the accepted method for assessing the abdominal artery, it has never been reported for evaluating the calcification visible in a chest CT. Then, to determine a useful, simple method applicable in routine COPD workups, we posited that calcification in an abdominal artery resembled that in a thoracic artery. Therefore, for each patient, we selected a slice with the most severe aortic wall calcification in thoracic artery, excluding the aortic arch, and a slice in which the aortic arch was longitudinally delineated. ACI (%) measurements in the thoracic artery and aortic arch were then recorded. When the findings were not agreed upon by consensus between two pulmonologists, the other pulmonologist finally determined its severity.
Statistical analyses
We used the Chi-squared test, paired t-test or Mann-Whitney test, as appropriate, to compare the two groups of patients. All data were subjected to Spearman’s rank correlation analysis. To determine independent factors associated with AA, we used logistic regression multivariable analysis. We also analyzed Akaike’s information criterion (AIC), which is a statistical value known to provide a superior fit, to obtain the most appropriate cut-off level (17,18). A smaller AIC value indicated a more reliable model for predicting the existence of AA. These analyses were performed with SPSS Version 21. Data were expressed as means with standard deviations (SD). For all statistical analyses, a P value less than 0.05 was considered significant.
Results
Baseline characteristics
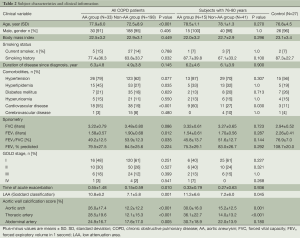
The mean age was 73.3 years (8.7) among the patients, of whom 94% were men, and 14% were current smokers. The mean FEV1 was 1.86 liters (83.7% of the predicted value), and patients whose COPD was classified as stage I, II, III, or IV, according to criteria of the GOLD guideline, comprised 59%, 26%, 13% and 2% of patients, respectively. The mean points of Goddard classification for lung damage were 7.4 (6.0). Of the 238 patients, 120 (52%) were given a long-acting inhaled muscarine receptor antagonist (LAMA); 91 (39%) received a long-acting inhaled β2-agonist (LABA), for 89 (39%) a corticosteroid inhalant was administered, 76 (33%) had a theophylline compound, and 28 (12%) received oxygen supplementation. As for co-morbidities, on the other hand, 149 patients (65%) had hypertension, 68 (29%) had hyperlipidemia, 42 (18%) had diabetes mellitus, 58 (24%) had cardiovascular disease other than AA, and 16 (7%) had cerebrovascular disease.
In six patients, abdominal AA had already been diagnosed and a repair operation was performed before this study. Meanwhile, our CT screening newly detected AA in 27 patients (Table 1), of whom 5 had thoracic AA, 18 had abdominal AA, 2 had common iliac AA, 1 had both thoracic and abdominal AA, and 1 had both abdominal and common iliac AA. Of 33 patients in this overall AA group, the maximal aortic diameter of 18 patients (55%) was less than 4 cm; another 7 (21%) had 4 to 5.5 cm, and 6 (18%) had more than 5.5 cm. Two patients whose AA was detected in our CT screening and who manifested an aortic diameter more than 5.5 cm underwent a repair operation after this study.
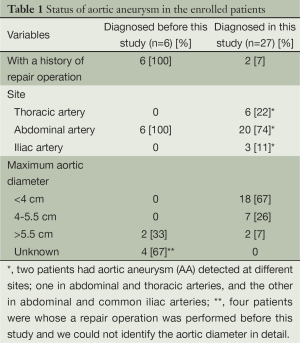
Full table
ACI in thoracic artery
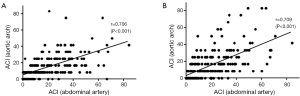
To assess the reliability of ACI in aortic arch and thoracic artery, we correlated those values with that of the previously established ACI in an abdominal artery (Figure 1). Indices for the current patients had strongly positive correlations with ACI in abdominal artery (ACI in aortic arch; r=0.709, P<0.001: ACI in thoracic artery; r=0.706, P<0.001).
Patients’ characteristics associated with AA
Comparisons of patients’ characteristics between the AA group (n=33) and non-AA group (n=198) designated here are shown in Table 2. The AA group were older (77.9 vs. 72.5 years, P<0.001), had a more excessive smoking status (pack-year, 77.4 vs. 63.8, P=0.032), higher Goddard score (10.8 vs. 7.1, P<0.001), elevated ACI in aortic arch, thoracic and abdominal arteries (26.0% vs. 12.2%, P<0.001; 28.5% vs. 12.1%, P<0.001; 24.9% vs. 17.6%, P=0.005, respectively) and far greater proportion of patients with cardiovascular disease (55% vs. 19%, P<0.001). Additionally, the AA group had a lower overall value for FEV1 (1.58 vs. 1.90 liters, P=0.012) and FEV1/FVC (49.2% vs. 53.9%, P=0.035) than the non-AA group. Gender, body mass index, co-morbidities except for cardiovascular disease, FEV1% predicted, and proportions of patients who were classified according to GOLD guideline were similar for both groups.
Full table
In contrast, however, age correlated negatively with body mass index (r=–0.184, P=0.005), FVC (r=–0.427, P<0.001), FEV1 (r=–0.341, P<0.001) and FEV1/FVC (r=–0.138, P=0.036), although greater age correlated positively with aortic calcification (P<0.001). Smoking status was also risk factor in both COPD and AA, and it correlated with aortic calcification as well as the FEV1% predicted and LAA (data not shown). Accordingly, our univariate analysis detected several characteristics of COPD shared with AA, but the influence of age differed notably between these two groups of patients.
Comparison of subjects with equivalent age
Based on our results, age was one of the most important factors contributing to AA in all patients with COPD. Accordingly, we next examined the clinical characteristics in individuals from both groups of comparable ages.
The ages yielding a 95% confidence interval (CI) in patients with AA was 75.8 to 80.1 years (range, 62-88 years). Therefore, we selected subjects of 76-80 years; 15 patients in AA group (78.5±1.1 years) and 41 patients in non-AA group (78.1±1.3 years) and compared data for the two groups (Table 2). As a result, Goddard scores and ACI in aortic arch and thoracic artery were higher in the AA group than in the non-AA group (Goddard score; 11.2 vs. 7.2, P=0.045: ACI in aortic arch; 30.0% vs. 15.2%, P= 0.001: ACI in thoracic artery; 36.1% vs. 14.0%, P<0.001). However, FEV1 was not significantly different (1.54 vs. 1.70 L, P=0.287; % predicted, 75.3% vs. 83.0%, P=0.292) in these two groups. In the multivariate analysis among those three significant parameters (data not shown), on the other hand, the two factors that were independently associated with the existence of AA were ACI in the thoracic artery [odds ratio (OR), 1.87; 95% CI, 1.08 to 3.25; P=0.026] and LAA (OR, 1.14; 95% CI, 1.01 to 1.28; P=0.033). However, ACI in aortic arch did not prove to be statistically significant (OR, 0.98; 95% CI, 0.51 to 1.86; P=0.943).
From those results, we plotted patients’ data according to ACI in the thoracic artery and Goddard scores, followed by calculating AIC to determine the most appropriate cut-off level (Figure 2). As a result, the smallest AIC value for the most appropriate cut-off levels of ACI in thoracic artery and Goddard scores were 25.0% and 9 (AIC =–9.14 and –5.31), respectively. Of 28 patients with Goddard score ≤9 and ACI ≤25.0%, only one patient had AA (3.6%), constituting a significantly lower prevalence in patients with low scores, i.e., Goddard score >9 or ACI >25.0% (50.0%, P<0.001).

Finally, to determine whether patients with COPD have a higher risk of AA than controls who are matched in age (76.8±4.5 years) and smoking status (packs/year, 87.3±22.7), we performed CT screening for 27 participants without COPD (Table 2). However, we did not detect AA in any of the latter participants (0%). Although the number of our control subjects was small, clearly the prevalence of AA in patients with COPD reached a statistically significant higher level than in our control group without COPD (P=0.002).
Discussion
In our prospective CT evaluation, we demonstrated for the first time the prevalence of AA and the specific characteristics of patients with AA among those who are afflicted with COPD. We found that (I) 33 of 231 COPD patients (14.3%) newly or previously diagnosed with AA represented a greater general prevalence of AA than previously reported (1-2%) [19]; (II) The age of 95% CI in the AA group was 75.8 to 80.1 years, and the prevalence of AA in patients of those ages was 26.8% (15 in 56 patients); (III) Comparison between those with COPD and separated into AA and non-AA groups of equivalent age indicated that LAA and ACI values evaluated by chest CT were higher in the AA group than those of the non-AA group, but FEV1 was not significantly different. Accordingly, our data indicate that COPD patients over 75 years of age have a high prevalence of AA and that severe lung destruction as well as aortic wall calcification is a risk factor for the acquisition of AA in these patients. Our data suggest that abdominal screening in addition to the routine COPD workup is a valuable asset in detecting AA early in the disease course of such patients and in averting AA-related adverse events.
AA involves the progressive dilatation of the aorta leading to a likelihood of tearing and high mortality rate typically associated with advanced age and atherosclerosis (19). Currently, the development of imaging mass spectrometry has elucidated the pathogenesis of AA. Tanaka et al. described adventitial stenosis and intimal hyperplasia of the vaso vasorum (VV) with the accumulation of abnormal lipid molecules in the AA sac and demonstrated that, in this state, the aortic wall of the AA sac was ischemic and hypoxic (20). That is an important risk factor for AA such as aging and smoking affect VV circulation ultimately causing the progression of AA (19,20). The systemic inflammation and hypoxic condition in COPD may also promote the contribution of abnormal VV circulation to AA.
Additionally, COPD shares these risk factors. Patients with AA had a higher prevalence of airway obstruction than age-matched control or patients with coronary artery disease (21). In an UPLIFT trial, furthermore, AA rupture was one of the common causes of death in spite of the fact that AA was not detected as a major co-morbidity (10). Our study also demonstrated that COPD patients, especially those who are over 75 years old, are at a higher risk of AA development than those without COPD or other cardiovascular co-morbidities. That is, these data suggest that a high degree of under-recognition of AA is likely in patients with COPD. Meanwhile, some epidemiologic studies suggest that diabetes is independently associated with a decreased rate of AA (22,23), but the proportion of patients with diabetes was not different between AA and non-AA group in our population. Whether this point is characteristic of patients with COPD remains unclear, we should re-evaluate it prospectively in multiple centers.
Here, ACI evaluation in chest CTs appeared to be a useful indicator for predicting the presence of AA in COPD patients. Most individuals aged >60 years have progressively enlarging deposits of calcium mineral in their major arteries (24). This vascular calcification reduces aortic and arterial elastance, which impairs cardiovascular hemodynamics; therefore ACI has been described as a valuable predictor of coronary heart disease (25,26). Methods for ACI evaluation have been reported, and those scores are independent factors for judgments on cardiovascular events (15,16,27). We assessed the aortic calcification in thoracic artery using ACI and confirmed its strong correlation with those scores previously reported (15,16). Accordingly, our data suggest that chest CT provides a good gauge of aortic calcification and cardiovascular risk.
This study had some limitations. First, the subjects in this study were located at a single center, and their number was smaller than in epidemiological studies. That is, we could not evaluate the interaction of LDL-C levels and statins, and the impact of the hypertensive medication such as beta-clockers and Losartan. However, our data were evaluated with state-of-the-art statistics and in correlation with previously established methods. Second, we did not assess the cost-effectiveness for detecting AA early in COPD patients. Because the incidence of AA is comparatively lower than other for cardiovascular diseases, the cost-effectiveness and indications for use should be evaluated. Finally, we considered that systemic inflammation in COPD could relate to the pathogenesis of AA, but we did not assess the systemic inflammatory markers (28). In addition, since our AA group included patients whose AA was previously diagnosed, there was a possibility that it caused a selection bias, and so the population was not representative from patients with COPD population and the prevalence was overestimated. Accordingly, our data including these points should be re-evaluated prospectively in multiple centers.
Conclusions
Our analysis revealed that the prevalence of AA is high in patients with COPD, suggesting a high degree of under-recognition of AA. Especially in patients with severe lung destruction and aortic calcification verifiable by chest CT, its prevalence is high and abdominal CT would be beneficial for detecting AA.
Acknowledgements
The authors thank Dr. Yasutaka Kawamura, M.D., Ph.D., Department of Radiology, Kameda Medical Center, for academic advice about the existence of AA. We also thank Phyllis Minick for excellent assistance in the review of English.
Authors’ contributions: KA had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis. KA and KN contributed to the concept and design of the study, acquisition of data, interpretation of data, and drafting and finalizing of the manuscript. AM and TK contributed to the concept and design of the study, and acquisition of data. DT contributed to the statistical analysis and interpretation of data. All authors read and approved the final manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (updated 2011). Bethesda, MD: National Heart, Lung and Blood Institute, World Health Organization, 2011.
- Curkendall SM, DeLuise C, Jones JK, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol 2006;16:63-70. [PubMed]
- Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest 2005;128:2640-6. [PubMed]
- Soriano JB, Visick GT, Muellerova H, et al. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest 2005;128:2099-107. [PubMed]
- Finkelstein J, Cha E, Scharf SM. Chronic obstructive pulmonary disease as an independent risk factor for cardiovascular morbidity. Int J Chron Obstruct Pulmon Dis 2009;4:337-49. [PubMed]
- Mannino DM, Thorn D, Swensen A, et al. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J 2008;32:962-9. [PubMed]
- Miller J, Edwards LD, Agustí A, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med 2013;107:1376-84. [PubMed]
- Lindholt JS, Heickendorff L, Antonsen S, et al. Natural history of abdominal aortic aneurysm with and without coexisting chronic obstructive pulmonary disease. J Vasc Surg 1998;28:226-33. [PubMed]
- Cronenwett JL, Murphy TF, Zelenock GB, et al. Actuarial analysis of variables associated with rupture of small abdominal aortic aneurysms. Surgery 1985;98:472-83. [PubMed]
- Celli B, Decramer M, Kesten S, et al. Mortality in the 4-year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009;180:948-55. [PubMed]
- Sharp DS. Reference values for lung function in the Japanese: recording of normal lung function from 11 institutes in Japan. Jpn J Thoracic Dis 1993;31:421-7.
- Goddard PR, Nicholson EM, Laszlo G, et al. Computed tomography in pulmonary emphysema. Clin Radiol 1982;33:379-87. [PubMed]
- Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg 2010;52:539-48. [PubMed]
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006;113:e463-654. [PubMed]
- Ohya M, Otani H, Kimura K, et al. Improved assessment of aortic calcification in Japanese patients undergoing maintenance hemodialysis. Intern Med 2010;49:2071-5. [PubMed]
- Yukawa S, Sonobe M, Tone Y, et al. Prevention of aortic calcification in patients on hemodialysis by long-term administration of vitamin E. J Nutr Sci Vitaminol (Tokyo) 1992;187-90. [PubMed]
- Akaike H. A new look at statistical model identification. IEEE Transactions on Automatic Control 1974;19:716-23.
- Wagenmakers EJ, Farrell S. AIC model selection using Akaike weights. Psychon Bull Rev 2004;11:192-6. [PubMed]
- Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature 2011;473:308-16. [PubMed]
- Tanaka H, Zaima N, Sasaki T, et al. Adventitial vasa vasorum arteriosclerosis in abdominal aortic aneurysm. PLoS One 2013;8:e57398. [PubMed]
- Sakamaki F, Oya H, Nagaya N, et al. Higher prevalence of obstructive airway disease in patients with thoracic or abdominal aortic aneurysm. J Vasc Surg 2002;36:35-40. [PubMed]
- Shantikumar S, Ajjan R, Porter KE, et al. Diabetes and the abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2010;39:200-7. [PubMed]
- Torsney E, Pirianov G, Cockerill GW. Diabetes as a negative risk factor for abdominal aortic aneurysm - does the disease aetiology or the treatment provide the mechanism of protection? Curr Vasc Pharmacol 2013;11:293-8. [PubMed]
- Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol 2004;24:331-6. [PubMed]
- Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography). Circulation 2007;115:402-26. [PubMed]
- Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation 2008;117:2938-48. [PubMed]
- Martino F, Di Loreto P, Giacomini D, et al. Abdominal aortic calcification is an independent predictor of cardiovascular events in peritoneal dialysis patients. Ther Apher Dial 2013;17:448-53. [PubMed]
- Eagan TM, Ueland T, Wagner PD, et al. Systemic inflammatory markers in COPD: results from the Bergen COPD Cohort Study. Eur Respir J 2010;35:540-8. [PubMed]


