Current status and evolution of robotic-assisted thoracic surgery in Germany—results from a nationwide survey
Introduction
The use of robotic-assisted surgery in routine clinical practice is becoming established in many surgical fields, including urology, visceral surgery, gynecology, and even thoracic surgery. The first description of a video-assisted thoracoscopic surgery (VATS) lobectomy with anatomical hilar dissection for cancer was published in 1992 (1), followed by the first reported series of robotic thoracoscopic surgery in 2002 (2). Currently, the da Vinci® Surgical System (Intuitive, Sunnyvale, CA, USA) is the only system available for thoracic surgery on the German market. The da Vinci Si® system has been available since 2009, and in 2015 the new generation da Vinci Xi® was introduced. Although there are multiple published case series of successful RATS procedures, there is still a lack of high-level evidence showing advantages of RATS compared to VATS. Nevertheless, there are several reasons why surgeons might initiate a RATS program (3). For example, it may be possible to improve the patient’s perioperative outcomes. Furthermore, the learning curve of robotic procedures is reported to be shorter compared to other minimally-invasive approaches (4,5); therefore, the transition from conventional surgery to RATS without the prior implementation of VATS may be easier. In addition, it may be presumed that the precision of the robotic system will be higher, especially with regard to mediastinal lymphadenectomy.
There is no existing data on the current status of RATS in Germany. In contrast, a nationwide survey was performed in visceral surgery in 2016, which showed a significant increase in robotic procedures within 41 surgical departments with an active robotic program (6). We conducted a survey to investigate the current status of RATS in Germany, and examine operation times, complications, conversion rates, length of hospital stay, and the development of RATS between 2013 and 2018.
Methods
We initiated a nationwide survey of all 22 German centers with an existing RATS program, using a standardized postal questionnaire. The number of operations per year, mean duration of surgery, docking time, length of hospital stay(s), conversions, chest tube duration, the start date of the RATS program, robot system used, OR capacity, and staplers and instruments used were recorded. All centers were able to report other procedures not displayed on the questionnaire, to gather complete data of the RATS procedures performed. The survey took place in the fourth quarter of 2018. Ethical approval for data collection was obtained. The statistical analysis was done with Prism 8 (GraphPad Software, CA, USA).
Results
We received 14 completed questionnaires from 22 centers (response rate 63%), including permission to use the data for further analysis. One responder was from a department with tremendous operative expertise in robotic thymectomy, resulting in a significant number (around 1,000) of robotic thymectomies in the German RATS collective. As the analysis of this cohort would have resulted in bias regarding the nationwide survey, the data was excluded from further statistical analysis. Twenty-three active robotic surgeons were reported in the responding centers. Eight centers did not answer our questionnaire, and whether they have a still-active robotic program remains unclear.
Currently, there are three different da Vinci® systems in clinical use for RATS. Six centers reported using da Vinci Xi®, four centers use da Vinci Si® and three use da Vinci X®. Nine centers have access to a da Vinci® Simulator, while a da Vinci® Proctor is part of the surgical team in three centers. There is an average of 1.8 RATS surgeons in every center.
Just one center reported the exclusive use of Intuitive surgical staplers for robotic lobectomy; two centers use conventional minimally-invasive staplers and Intuitive staplers, and nine centers prefer Covidien Staplers. For RATS lobectomy, half of the centers prefer using three robotic arms, while the other half uses four arms. For robotic lobectomy, most centers prefer a Maryland bipolar forceps on the right and a Cadiere forceps on the left. Other standard instruments are the curved bipolar dissector for the right arm and the fenestrated bipolar for the left arm. Most centers with an established 4-arm technique use a thoracic grasper for the third arm.
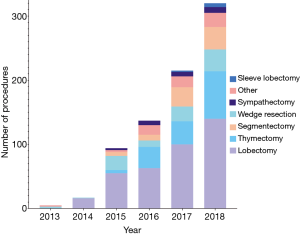
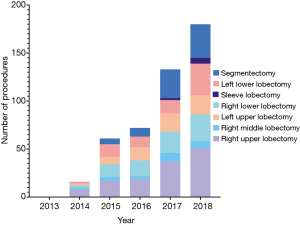
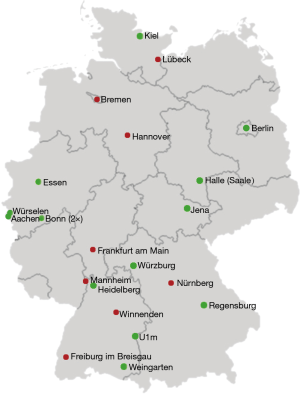
The geographic distribution of centers with an active robotic program in Germany is shown in Figure 1, while the development of RATS in Germany is illustrated in Figure 2. The first recorded robotic sleeve lobectomy was reported in 2017. Further distribution of robotic anatomic lung resections is shown in Figure 3.
There was a yearly increase in active centers, with an average increment of two centers per annum. The most striking development is the increase in the number of segmentectomies; six segmentectomies were recorded in 2016, which increased to 35 procedures in 2018.
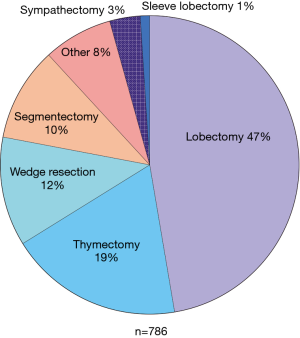
Excluding the series of around 1,000 RATS thymectomies of a single center, there were 786 recorded RATS procedures. The most common procedure was the RATS lobectomy (372 cases, 47%), followed by thymectomy (19%), wedge resection (12%), segmentectomy (10%), and sympathectomy (3%). Other RATS procedures, such as lymphadenectomy, decortication, resection of a mediastinal tumor, biopsy, and phrenoplication, had a share of 8%. In total, eight robotic sleeve lobectomies were recorded. The distribution of RATS procedures throughout the study period is shown in Figure 4. The predominate diagnosis for RATS was NSCLC. In the high-volume centers’, tumors with a diameter of >8 cm and T4-staging were resected via thoracotomy. In the low-volume centers a further patient selection has to be assumed due to the learning curve and lack of OR capacities, personal opinion of the surgeon and patient wish.
Throughout the study period, three centers performed >150 RATS procedures in total, one center carried out 100-150 procedures, two centers 50–100 procedures, and eight centers <50 procedures (Table 1). In 2018, three centers performed <50 RATS procedures, three centers 25–50 procedures, and eight centers <25 procedures.
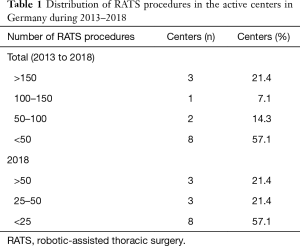
Full table
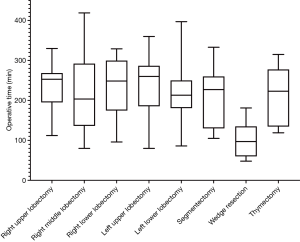
The mean operative time was 245 minutes (range, 80–419 minutes) for a RATS lobectomy, 219 minutes (range, 105–333 minutes) for a segmentectomy, 97minutes for a wedge resection, and 195 minutes for a thymectomy. The average incision-to-suture time for the eight reported sleeve lobectomies was 306 minutes. The distribution of the mean operative times is shown in Figure 5.
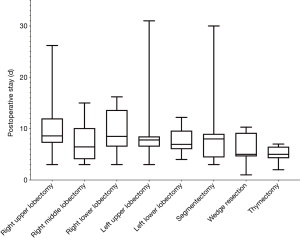
The mean duration of postoperative stay was 8 days (range, 3–31 days) after a RATS lobectomy. The mean duration of postoperative stay was 7 days (range, 3–30 days) after segmentectomy and 5 days (range, 2–7 days) after robotic thymectomy. The distribution of the duration of hospital stay is shown in Figure 6.
The overall conversion rate was 6.7%. The distribution of conversion rates for different RATS procedures is shown in Table 2. There were significant differences in conversion rates, depending on the procedure. The high conversion rate of 17.5% (11 out of 63 procedures) for the left upper lobectomy is striking. In contrast, the conversion rate was 0% for sleeve lobectomy and sympathectomy. The primary reasons reported for conversion were bleeding, unfavorable anatomy, adhesions, inability to detect small tumors, and the unexpected need for an angioplasty resection.
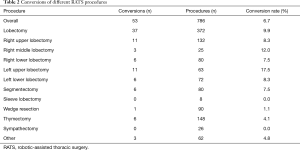
Full table
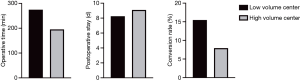
Centers with an experience of >100 RATS-lobectomies in total were defined as high-volume centers (n=2), whereas all other centers were defined as low-volume centers. Figure 7 shows the mean operative time, postoperative stay, and conversion rate for RATS-lobectomy, separated by the experience of the centers. In the high-volume centers, lower operative times and conversion rates were reported, whereas the length of postoperative stay was similar.
The mean docking time was 24 minutes for the first 15 RATS procedures, and nine minutes for the next 15 procedures. This reduction of docking time can be achieved by training of the OR-team and standardization. The mean number of OR days was 1.9 (range 0.5–5) days per week. All surveyed centers plan to further expand RATS, with a lack of OR capacity being a frequent impediment.
Discussion
Our survey provides the first published data examining the status of RATS surgery in Germany. Overall, RATS is becoming more established in everyday clinical practice. Since 2013, there has been a significant increase in the number of operations, active centers, and trained RATS surgeons. Yet RATS is still at the beginning of its development. In the United States, for example, 7,600 robotic lobectomies had been performed up to 2017 (7). In Italy, the development of RATS began in 2011, quickly followed by the publication of a large series of robotic lobectomies (8). Our data indicates that further expansion of the robotic program in Germany has been limited by the lack of OR capacity; all centers who responded plan to further expand their RATS program.
Our data shows a significant increase of RATS in Germany. This might be due to the well-known technical advantages of robotic-assisted surgery. It can be assumed that surgeons are interested in this new field of surgery. Additionally, a shorter learning curve of robotic lobectomy compares to VATS-lobectomy was described (9), leading to the assumption that a transition from open to RATS might be easier to achieve. Furthermore, surgeons might assume that robotic surgery leads to the ability to take care of more complicated cases.
Our results are restricted due to the relative low response rate of 63%, possibly due to recent changes in the leading positions of the corresponding centers. We obtained information about the active centers in Germany by communication with Intuitive and personal conversations at scientific meetings. Furthermore, two authors are official proctors for RATS. Because of that, we are confident that all active RATS-centers received a questionnaire. However, it must be considered that the non-responders no longer have an active robotic thoracic program, although ultimately this remains unclear.
The feasibility of RATS has been demonstrated in several studies (8,10,11). Postoperative complications and perioperative mortality have previously been described, with no significant differences between RATS and VATS (12). An overall conversion rate of 6.7% was reported by our responders, whereas conversion rates of 9–10% have been reported in the literature (7,13). In our own centers, we have observed that our conversion rates have decreased significantly with increasing experience in a robotic program. The main reasons for conversions were bleeding and adhesions; most cases of bleeding can be controlled initially using a robotic approach, followed by controlled conversion if necessary (14). In our survey data, an exceptionally high conversion rate of 17.5% was reported for upper left lobectomy, which is considered the most challenging lobectomy due to anatomical reasons, in our own experience this rate can be further reduced with increasing experience in RATS.
In our survey data, the mean operative time for a robotic lobectomy was 245 minutes. Robotic segmentectomies were reported with a shorter operative time of 219 minutes, consistent with published data (15). Additionally, it has to be assumed that segmentectomies are a later step in the establishment of a robotic program. For VATS lobectomy, the median procedure length was 130 minutes when performed by an experienced surgical team (16). However, RATS in Germany is still at the beginning of its evolution, whereas VATS lobectomy has a two-decade long tradition with many highly experienced centers. There is some data to determine the spread of VATS in Germany. In 2016, 122/227 (53.7%) german thoracic surgery centers had some experience in VATS-lobectomy, whereas 77/122 (63.1%) of those centers performed cumulative <50 procedures (17). The ratio of VATS-lobectomies in Germany is given with 50% in experienced centers, with increasing rates of VATS and decreasing rates of conventional thoracic surgery (18). The number of RATS-lobectomies must be estimated much lower. Within our own centers, operative times have been reduced to the level of open surgery with increasing experience in RATS lobectomy. There is some published data available for robotic lobectomy, with a reported operative time of 132 minutes in an experienced center (19).
The mean postoperative stay after RATS lobectomy was eight days, which is long compared to international results (20). This is mainly due to a different healthcare system, especially in the outpatient sector. In Germany, the healthcare system is focused on postoperative inpatient healthcare, with a lack of adequate structures for outpatient postoperative follow-up. Besides that, the not completed learning curve in all centers might lead to a more cautious postoperative management for the first cases.
Little is known about the oncologic results after using RATS. In a recent study, there was no difference in survival rates, whereas the lymph node yield was higher using the robotic approach (21). In German thoracic surgery, there is due to the high cost of an robotic approach a historical trend in favor of VATS lobectomy, with its evolution to an uniportal approach (22,23). However, no data-based analysis of these questions exists. Whether VATS or thoracotomy is a better approach has been debated intensively and remains controversial (24). In particular, the quality and completeness of thoracic lymphadenectomy in the video-assisted approach remains unclear. Nodal upstaging is regarded as a quality measurement concerning complete lymph node dissection in oncologic lung surgery. There is some evidence for higher rates of nodal upstaging in favor of thoracotomy compared to VATS (25), for several reasons. For example, performing a complete lymph node dissection using VATS is more challenging due to limited maneuverability in the narrow mediastinal space. Thus, there might be an advantage in favor of robotic hilar dissection. The main disadvantage of robotic surgery is the lack of haptic feedback, it has been shown that a robotic expert can overcome this lack, probably because of an evolving optical feedback (26). There is some evidence that a robotic approach is superior to VATS lobectomy regarding nodal upstaging (27). Differences in survival rates were not recorded, so the clinical relevance of these findings remains unclear (28). Nevertheless, the broad application of VATS in Germany might be one of the main reasons why the development of RATS lags behind other European countries and the United States.
Despite the potential advantages of RATS for mediastinal lymphadenectomy, it is an ideal tool for parenchyma-sparing surgery. Even extended resections with broncho- or angioplasty are reported in our survey, with low morbidity and mortality, and can be carried out safely (14). In our data, a significant increase in the rate of robotic segmentectomies was observed, which is also a concept in parenchyma-sparing lung surgery.
Initially, RATS was criticized for the high initial cost of the system and the reusable instruments, with a described cost difference of nearly US$5,000 for a robotic lobectomy in an American study from 2014 (20). New technologies always suffer from an initially high financial burden, due to the development costs from the manufacturer. Furthermore, the manufacturer has an ongoing financial burden regarding training of surgeons and proctoring programs. By expanding the technology, one can expect a decline in costs over the coming years. Furthermore, the approval of competitive products is eagerly awaited to challenge the monopolistic status of the current manufacturer, as further innovations should contribute to a decrease in the cost of robotic surgery. After an American nationwide survey, a RATS curriculum was proposed to be integrated into existing thoracic surgery residency programs (29). In Germany, there is currently no structured robotic training program for residents in thoracic surgery.
The evolution of RATS is still ongoing, encouraged by new surgical techniques and technical improvements. The next step might be the introduction of a single-port robotic platform to the German market. The first experience with this innovative platform was published in a urological case series; the adoption for RATS is probably the next step in its evolution (30,31). In particular, considering the promising results with uniportal lobectomy, this might combine the advantages of both approaches (22).
Conclusions
More high-level and high-quality evidence in the evolving field of RATS is needed. For that reason, the formation of an organized structure of all German RATS surgeons and centers is necessary to gather complete data of RATS in Germany and internationally. Ideally, a RATS register should be founded to enable multicenter studies, prospective data collection, and transparent documentation. With the present study, we have tried to contribute to this exigency by connecting the German RATS centers and presenting the current status and evolution of RATS in Germany.
Acknowledgments
None.
Footnote
Conflicts of Interest: JH Egberts, JC Rückert are working as a proctor for intuitive. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7. [PubMed]
- Melfi FMA, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Louie BE, Farivar AS, Aye RW, et al. Early Experience With Robotic Lung Resection Results in Similar Operative Outcomes and Morbidity When Compared With Matched Video-Assisted Thoracoscopic Surgery Cases. Ann Thorac Surg 2012;93:1598-604. [Crossref] [PubMed]
- Moore LJ, Wilson MR, Waine E, et al. Robotic technology results in faster and more robust surgical skill acquisition than traditional laparoscopy. J Robot Surg 2015;9:67-73. [Crossref] [PubMed]
- Cheufou DH, Mardanzai K, Ploenes T, et al. Effectiveness of Robotic Lobectomy—Outcome and Learning Curve in a High Volume Center. Thorac Cardiovasc Surg 2019;67:573-7. [Crossref] [PubMed]
- Kissler HJ, Bauschke A, Settmacher U. First national survey on use of robotics for visceral surgery in Germany. Chir Z Alle Geb Oper Medizen 2016;87:669-75. [Crossref] [PubMed]
- Arnold BN, Thomas DC, Narayan R, et al. Robotic-Assisted Lobectomies in the National Cancer Database. J Am Coll Surg 2018;226:1052-62.e15. [Crossref] [PubMed]
- Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): Long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. [Crossref] [PubMed]
- Solinas M, Novellis P, Veronesi G. Robotic is better than VATS? Ten good reasons to prefer robotic versus manual VATS surgery in lung cancer patients. Video-assist Thorac Surg 2017;2:60. [Crossref]
- Kent M, Wang T, Whyte R, et al. Open, Video-Assisted Thoracic Surgery, and Robotic Lobectomy: Review of a National Database. Ann Thorac Surg 2014;97:236-42. [Crossref] [PubMed]
- Wei S, Chen M, Chen N, et al. Feasibility and safety of robot-assisted thoracic surgery for lung lobectomy in patients with non-small cell lung cancer: a systematic review and meta-analysis. World J Surg Oncol 2017;15:98. [Crossref] [PubMed]
- Louie BE, Wilson JL, Kim S, et al. Comparison of Video-Assisted Thoracoscopic Surgery and Robotic Approaches for Clinical Stage I and Stage II Non-Small Cell Lung Cancer Using The Society of Thoracic Surgeons Database. Ann Thorac Surg 2016;102:917-24. [Crossref] [PubMed]
- Glenn ZF, Zubair M, Hussain L, et al. Comparison of pulmonary lobectomies using robotic and video-assisted thoracoscopic approaches: results from 2010 - 2013 National Inpatient Sample. J Cardiovasc Surg (Torino) 2019;60:526-31. [Crossref] [PubMed]
- Egberts JH, Möller T, Becker T. Robotic-Assisted Sleeve Lobectomy Using the Four-Arm Technique in the DaVinci Si® and Xi® Systems. Thorac Cardiovasc Surg 2019;67:603-5. [Crossref] [PubMed]
- Toker A, Ayalp K, Uyumaz E, et al. Robotic lung segmentectomy for malignant and benign lesions. J Thorac Dis 2014;6:937-42. [PubMed]
- Swanson SJ, Herndon JE, D’Amico TA, et al. Video-Assisted Thoracic Surgery Lobectomy: Report of CALGB 39802—A Prospective, Multi-Institution Feasibility Study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Reichert M, Gohlke AB, Augustin F, et al. Video-assisted thoracoscopic anatomic lung resections in Germany—a nationwide survey. Langenbecks Arch Surg 2016;401:877-84. [Crossref] [PubMed]
- Hofmann HS. VATS - technique and indications. Chirurg 2015;86:711-21. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Swanson SJ, Miller DL, McKenna RJ, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: Results from a multihospital database (Premier). J Thorac Cardiovasc Surg 2014;147:929-37. [Crossref] [PubMed]
- Yang HX, Woo KM, Sima CS, et al. Long-term Survival Based on the Surgical Approach to Lobectomy For Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg 2017;265:431-7. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal Video-Assisted Thoracoscopic Lobectomy: Two Years of Experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Grallert M, Uhlmann D, Bartels M, et al. VATS-Lobektomie - Ein Standardverfahren in der Therapie des nicht kleinzelligen Lungenkarzinoms im Stadium I? Zentralblatt für Chirurgie 2013;138:S40-4. [Crossref] [PubMed]
- Gopaldas RR, Bakaeen FG, Dao TK, et al. Video-Assisted Thoracoscopic Versus Open Thoracotomy Lobectomy in a Cohort of 13,619 Patients. Ann Thorac Surg 2010;89:1563-70. [Crossref] [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph Node Evaluation by Open or Video-Assisted Approaches in 11,500 Anatomic Lung Cancer Resections. Ann Thorac Surg 2012;94:347-53. [Crossref] [PubMed]
- Meccariello G, Faedi F, AlGhamdi S, et al. An experimental study about haptic feedback in robotic surgery: may visual feedback substitute tactile feedback? J Robot Surg 2016;10:57-61. [Crossref] [PubMed]
- Wilson JL, Louie BE, Cerfolio RJ, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg 2014;97:1901-6; discussion 1906-7.
- Licht PB, Jørgensen OD, Ladegaard L, et al. A National Study of Nodal Upstaging After Thoracoscopic Versus Open Lobectomy for Clinical Stage I Lung Cancer. Ann Thorac Surg 2013;96:943-9; discussion 949-50. [Crossref] [PubMed]
- Raad WN, Ayub A, Huang CY, et al. Robotic Thoracic Surgery Training for Residency Programs: A Position Paper for an Educational Curriculum. Innovations 2018;13:417-22. [Crossref] [PubMed]
- Ramirez D, Maurice MJ, Kaouk JH. Robotic Single-port Surgery: Paving the Way for the Future. Urology 2016;95:5-10. [Crossref] [PubMed]
- Kaouk JH, Haber GP, Autorino R, et al. A Novel Robotic System for Single-port Urologic Surgery: First Clinical Investigation. Eur Urol 2014;66:1033-43. [Crossref] [PubMed]