Analysis on minimally invasive diagnosis and treatment of 49 cases with solitary nodular ground-glass opacity
Introduction
In recent years, with the improvement of people’s health awareness and the development of imaging technology as well as the application of lung cancer screening, pulmonary ground-glass opacity (GGO), especially solitary nodular ground-glass opacity (SNGGO), has attracted more and more attention from clinicians due to its difficulty in diagnosis through non-invasive examination. In literature, GGO is used to describe a finding on high-resolution CT (HRCT) of the lung in which “hazy increased attenuation of the lung with preservation of bronchial and vascular margins” is seen. It could be caused by partial filling of air spaces, interstitial thickening, partial collapse of alveoli, normal expiration, or increased capillary blood volume. It may be associated with an air bronchogram. The literature further pointed out that GGO should not be confused with “consolidation”, in which bronchovascular margins are obscured. Moreover, GGO has the characteristics of small size (usually <20 mm in diameter), low density, and atypical morphology at the same time (1). This study is designed to investigate the treatment approach and prognosis of SNGGO. Forty-nine cases of SNGGO admitted by our hospital from June 2011 to February 2014 were retrospectively reviewed, and the experiences in treating the 49 cases are reported here in reference with literatures.
Patients and methods
Patients
We retrospectively studied patients with pulmonary nodules who met the following criteria: (II) HRCT showed the lesions were GGO; (II) lesion of ≥5 mm in diameter; (III) GGO proportion of ≥50%; and (IV) the lesion persisted or continued growing after 2 weeks’ anti-inflammatory treatment.
Surgical procedure
After adequate preoperative preparation, patients received surgical treatment. For those with small or deeply located lesion, methylene blue tattooing or hookwire positioning was performed before surgery. Double-lumen endotracheal intubation and intravenous general anesthesia were performed and three incisions were made: a 3 cm incision in the 3rd or 4th intercostal space at the anterior axillary line as the primary operating hole; a 1 cm incision in the 7th or 8th intercostal space at the middle axillary line as the observation hole; and a 1 cm incision in the 7th or 8th intercostal space at the infrascapular line as the auxiliary operating hole. After that, a wedge resection was made by Endo-GIA stapler, and the specimens were put in the endobag and removed from the main port. The GGO were resected from the specimens, marked with suture, and diagnosed by intraoperative frozen section. If the GGO was benign, the chest was closed. If it was malignant, lobectomy plus lymph node dissection was performed; for those who cannot tolerate, lung segment resection or pulmonary wedge resection was performed.
Statistical analyses
SPSS13.0 software is used to analyze the data. Chi-square test is adopted to evaluate the correlation between malignancy and patient age, gender, lesion size and location. P<0.05 is recognized as the level of significance.
Results
General information
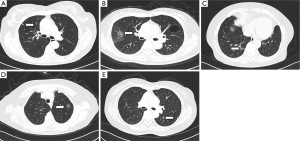
Among patients admitted by our hospital from June 2011 to February 2014, 49 met the inclusion criteria and were included for further analysis in this study. The 49 cases included 12 male and 37 female patients aged 53.54±12.57 years. Of these patients, 23 showed no symptom and the GGO were detected during physical checkup; the other 26 patients were hospitalized due to varying degrees of cough, chest pain, chest discomfort or other symptoms. After 2 weeks’ anti-inflammatory treatment, the lesions persisted or continued growing. The size of the GGO in this study ranged from 5×7 to 15×18 mm. Specifically, 20 cases was <10 mm in diameter, other 29 cases was 10-20 mm, and none of them was >20 mm. Seventeen lesions were located in the upper right lung, 3 in middle right, 7 in lower right, 12 in upper left, and 10 in lower left (Figure 1A-E). None of the patients had pathological diagnosis before the surgery.
Surgical treatment
Intraoperative frozen section showed 43 cases of malignancy (87.75%), among which 36 cases received VATS lobectomy and seven received simple wedge resection or pulmonary segment resection due to poor lung function. No metastasis was detected during lymph node dissection or sampling. For six non-malignant cases, two cases were atypical adenomatous hyperplasia (AAH) (4.09%), and four cases were benign and received lesion resection only. No operative deaths occurred. Four patients had pulmonary infection after surgery but were all cured after proper treatment.
Postoperative pathological diagnosis
Postoperative pathological analysis results were in agreement with intraoperative frozen section results. For details, 43 cases were primary adenocarcinoma or micro-infiltrating adenocarcinoma; two cases were AAH of alveolar epithelia; one case was hyaline nodules; one case was chronic inflammation of lung tissue combined with focal vasodilation, hemorrhage and interstitial fibrosis; one case was normal lung tissue; and one case was focal angiogenesis and epithelial hyperplasia. Postoperative pathological staging for 43 malignant cases were all Ia.
Postoperative outcome
The patients were followed up for 1 to 30 months with a median follow-up time of 19 months. Neither recurrence nor metastasis was detected during the follow-up period.
Correlation analyses
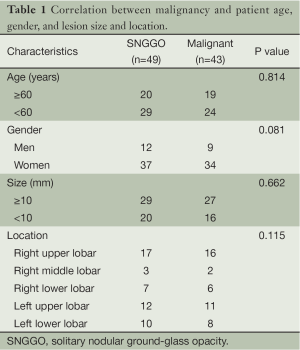
Correlation between malignancy and patient age, gender, lesion size and location was analyzed by chi square test and the results were shown in Table 1. No correlation was found between malignancy and patient age, gender, lesion size or location.
Full table
Discussion
Pulmonary GGO is one of the hot spots in clinical research of lung in recent years. Pulmonary GGO can be clouding or nodular, solitary or multiple. SNGGO is often found in lung cancer screening. Due to its high rate of malignancy and difficulty in diagnosis, it was much more studied by radiologists in the initial stage (2-5). According to whether there is a solid component within the composition, lesions are divided into pure GGO (pGGO) and mixed GGO (mGGO). In this study, most of the cases are pGGO. Because only three cases of GGO containing solid component were adenocarcinoma, the proportion of the cases was very small, and the analysis results had no statistical significance, the further discussion was not performed. In the future, when this type cases are adequate, the analysis of this type cases will be performed separately.
Currently there is still no non-invasive method that can accurately diagnose SNGGO. As reported by Infante et al. (6), PET has low sensitivity for nodular GGO (25%), therefore is not considered as a routine diagnostic method. As for invasive diagnostic methods, there are mainly two types:
- CT-guided lung biopsy: for nodular GGO that persists after antibiotic treatment, CT-guided lung biopsy can identify patients that require surgical resection. As reported by Kim et al. (7), this method has few complications and has a positive predictive value of 97% and a negative predictive value of 75%, so it is commonly used for the identification of nodular GGO. As reported by Godoy et al. (8), CT-guided lung biopsy has an overall diagnostic rate of only 64.6%, and the rate is even lower for lesions with a diameter ≤10 mm and GGO >50%, which is about 35.2%. It was reported that for patients diagnosed with adenocarcinoma in biopsy, treatment with radiofrequency ablation achieved nearly 100% 1-, 3-, and 5-year survival (9). This outcome is comparable to that of surgical resection, but only in the context of clear diagnosis. A negative biopsy result cannot rule out the possibility of malignancies; missed diagnosis and misdiagnosis are common.
- VATS-guided wedge resection: intraoperative rapid frozen section can be made for clear diagnosis, so that diagnosis and treatment of GGO can be performed simultaneously. All malignant cases in this study were adenocarcinoma. It has been reported that for GGO diagnosed as adenocarcinoma, minimally invasive wedge resection is superior to lobectomy because the former can better reserve lung function (10). Limited resection (e.g., a wedge resection or segmentectomy) can greatly reserve pulmonary function, reduce trauma, achieve quick recovery, reduce complications, especially in patients with poor pulmonary function, as well as reduce economic burden for patients at the same time. However, limited excision also has some limitations: extrusion could cause metastasis in the process of palpation or resection, and inadequate resection margin may lead to recurrence. Therefore, regular follow-up for imaging examination and treatment is highly recommended. Based on our experience, we propose that for patients with tolerability in lung function, especially young patients, lobectomy shall be recommended. However, there’s currently no solid evidence to support this idea, and long-term follow-up of large number of cases needs to be conducted. For GGO near the hilum and larger than 10 mm in diameter, lobectomy is considered if lung function permits (11). But if the diameter of the lesion is 5-10 mm, we suggest regular review of the case, and if the solid component is more than 50%, lobectomy is recommended. For GGO hard to be found during surgical procedure, the patient’s HRCT was pored over by the surgeon in order to locate the lesion, and the lung tissue containing GGO according to the corresponding position of the CT image was resected for frozen section analysis. In some cases, multiple wedge resections were needed and continuous frozen section analysis was performed by surgeon and pathologist collaboratively until the lesion was found.
Some scholars suggested that the size of GGO lesion is of significance in the diagnosis of malignancy (12). In the current study, the size of the 49 cases of GGO ranged from 5×7 to 15×18 mm. Sixteen of the 20 cases that had a lesion of 5-10 mm in diameter were malignant, accounting for 80%; and 27 of the 29 cases that had a lesion of 10-20 mm in diameter were malignant, accounting for 93%. It seems that bigger lesion size indicates higher rate of malignancy, but exact probability analysis showed no significant correlation between the two (P=0.662), which is in accordance with the results reported by Li et al. (13). Therefore, cautions shall be taken when taking lesion size into diagnostic consideration. For lesions less than 5 mm in diameter, we recommend continuous follow-up for these cases. If the lesion has no change, HRCT should be taken every three months for the 1st year and every 6 months after 1 year. If expansion of the lesion or obvious solid component is observed, surgical resection is recommended.
As for the location of the lesion, although in the current study, it seems that GGO tends to occur in the upper lobe of the lung, it doesn’t help identify the nature of the lesion either (P=0.115).
In this study, all malignancies were adenocarcinoma. Nine of the 12 male patients (75%) and 34 of the 37 female patients (92%) were malignant. Female seems to have a higher rate of malignancy than male, but chi-square test showed no significant difference between them (P=0.08). The negative result may be due to the insufficient number of patients in this study.
Two cases of AAH of alveolar epithelium in this study had a >10 mm lesion, and received lung segment resection. World Health Organization has suggested that AAH is a putative precursor to lung adenocarcinoma, the initial stage in the process of adenoma—bronchioloalveolar carcinoma (BAC) (BAC, currently renamed as adenocarcinoma in situ or AIS)—invasive adenocarcinoma. As for whether non-cancerous solitary AAH should be surgically resected, no consensus has been reached at present. But if the patient has high risks of lung cancer, minimally invasive surgery might be worthy.
Generally speaking, nodules stable for over 2 years or calcified need no further treatment. However, there’re cases where the nodule has been stable for over 2 years but turned out to be malignant when surgically resected. When nodular GGO is found in lung CT scan, imaging features including the density, size of the lesion and existence of solid components should be carefully analyzed. Lung CT scan showing lobulation, speculation, vascular convergence, etc. should be actively examined and treated. Specialty Committee of Lung Cancer has suggested in Clinical Guidelines for the Diagnosis and Treatment of Lung Cancer that “solitary nodular lesions of the lung should receive thoracoscopic resection of the tumor or thoracotomy for tumor resection plus intraoperative frozen section, so as to perform diagnosis and treatment simultaneously” (14). Some Chinese experts suggested that for micro lesion of the lung that persisted after 2 weeks of anti-inflammatory therapy or 1 month of anti-tuberculosis therapy, minimally invasive surgery should be performed for definitive diagnosis and complete recovery (15).
Conclusions
In summary, according to the results of our research, SNGGO has high tendency of canceration. Prognoses after surgical resection in patients with malignant SNGGO nodules are favorable. Neither local recurrence nor metastases occurred irrespective of nodule number, size, surgical method, presence of size change before surgical removal, or histopathological diagnosis. VATS-guided resection of tumor plus intraoperative frozen section with preservation of lung volume and adequate follow-up imaging studies are recommended for both diagnosis and treatment. Since the number of cases is limited and the follow-up time is relatively short in this study, long-term prognosis of SNGGO requires further investigation.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81102200/H2610).
Disclosure: The authors declare no conflict of interest.
References
- Collins J, Stern EJ. Ground-glass opacity at CT: the ABCs. AJR Am J Roentgenol 1997;169:355-67. [PubMed]
- Kim HY, Shim YM, Lee KS, et al. Persistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisons. Radiology 2007;245:267-75. [PubMed]
- Nakata M, Saeki H, Takata I, et al. Focal ground-glass opacity detected by low-dose helical CT. Chest 2002;121:1464-7. [PubMed]
- Henschke CI, Yankelevitz DF, Mirtcheva R, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 2002;178:1053-7. [PubMed]
- Lee HJ, Goo JM, Lee CH, et al. Nodular ground-glass opacities on thin-section CT: size change during follow-up and pathological results. Korean J Radiol 2007;8:22-31. [PubMed]
- Infante M, Lutman RF, Imparato S, et al. Differential diagnosis and management of focal ground-glass opacities. Eur Respir J 2009;33:821-7. [PubMed]
- Kim TJ, Lee JH, Lee CT, et al. Diagnostic accuracy of CT-guided core biopsy of ground-glass opacity pulmonary lesions. AJR Am J Roentgenol 2008;190:234-9. [PubMed]
- Godoy MC, Naidich DP. Subsolid pulmonary nodules and the spectrum of peripheral adenocarcinomas of the lung: recommended interim guidelines for assessment and management. Radiology 2009;253:606-22. [PubMed]
- Kodama H, Yamakado K, Hasegawa T, et al. Radiofrequency ablation for ground-glass opacity-dominant lung adenocarcinoma. J Vasc Interv Radiol 2014;25:333-9. [PubMed]
- Lee HY, Lee KS. Ground-glass opacity nodules: histopathology, imaging evaluation, and clinical implications. J Thorac Imaging 2011;26:106-18. [PubMed]
- Park JH, Lee KS, Kim JH, et al. Malignant pure pulmonary ground-glass opacity nodules: prognostic implications. Korean J Radiol 2009;10:12-20. [PubMed]
- Lin JH, Jiang HW, Wang WH. Study of the histological and radiologic characteristics of pulmonary focal ground-glass opacity. Radiol Practice 2006;21:667-9.
- Li F, Sone S, Abe H, et al. Malignant versus benign nodules at CT screening for lung cancer: comparison of thin-section CT findings. Radiology 2004;233:793-8. [PubMed]
- Alzahouri K, Velten M, Arveux P, et al. Management of SPN in France. Pathways for definitive diagnosis of solitary pulmonary nodule: a multicentre study in 18 French districts. BMC Cancer 2008;8:93. [PubMed]
- Gossot D, de Kerviler E, Paladines G, et al. Thoracoscopic approach in pulmonary nodules: a prospective evaluation of a series of 120 patients. Rev Mal Respir 1997;14:287-93. [PubMed]


