The association between plasma fibrinogen levels and lung cancer: a meta-analysis
Introduction
Lung cancer was considered to be the leading cause of cancer-related mortality (1). There were 1.8 million new lung cancer cases and 1.6 million cancer-related deaths worldwide (2). In spite of great advances in diagnosis and therapy, 5-year survival rates are only 18% for lung cancer (3). Independent prognostic factors, including disease stage, performance status, age, sex, weight loss and KRAS mutation, have been identified (4-8). However, there is a lack of blood biochemical indicators for the prognosis and clinicopathological characteristics in patients suffering from lung cancer.
Fibrinogen, a easily measured biochemical indicator, is a kind of the principal acute phase proteins generated by the liver (9). The activation of coagulation and fibrinolysis is encountered among malignancy and about 90% of cancer patients with metastatic disease and 50% of cancer patients have abnormal coagulation parameters (10). Moreover, high plasma fibrinogen level is frequently observed in cancer patients and influence metastatic potential (11,12). Several meta-analysis indicated that elevated plasma fibrinogen associated with poor prognosis (overall survival, OS; progression-free survival, PFS; disease-free survival, DFS) in epithelial ovarian, digestive cancer (13,14). Moreover, elevated plasma fibrinogen level in lung cancer implied short survival (15). Then, anticoagulant therapy would be valuable for patients with lung cancer. However, conflicting data were reported (16,17).
Therefore, we conducted a meta-analysis to assess the prognostic significance of fibrinogen in patients with lung cancer.
Methods
Search strategy
A systemic search in PubMed, Web of science, EMBASE and the Cochrane Central Register of Controlled Trials databases were employed to identify all articles published up to September 2018, using the items “fibrinogen”, “hyperfibrinogen”, “fibrinogenemia”, “predictive”, “recurrence”, “relapse”, “prognosis”, “survival”, “lung cancer” and “lung neoplasms”. In addition, we manually searched abstracts of the World Lung Cancer Conference, the American Society of Clinical Oncology (ASCO) and the European Society of Medical Oncology (ESMO) to identify unpublished studies.
Selection criteria
Eligible studies included in the meta-analysis had to meet the following criteria: (I) assessment of the association of plasma fibrinogen and lung cancer prognosis or clinicopathological characteristics; (II) the value of plasma fibrinogen detection before chemotherapy; (III) only including the most recent and informative studies if duplicate articles were from the same participants; (IV) full text articles in English or Chinese language were retained.
Data extraction
Data from the included studies were extracted independently by two investigators (K Zhang and Y Xu). The information, including first author, year of publication, patient source, number of patients, histology, disease stage, cut-off value and outcomes, was extracted. Two reviewers (K Zhang and Y Xu) determined study eligibility independently and any disagreements were solved after discussion.
Quality assessment
The STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) checklist was used to assess the quality of these studies for cohort studies (18). The overall score assessed the following dimensions of methodology, grouped into eight main categories: (I) hypothesis and/or objective(s) stated; (II) tumor stage clearly described; (III) clear description of eligibility criteria; (IV) patients with inflammatory disease or coagulation disorders excluded; (V) predictors and outcome(s) clearly predefined; (VI) confounders considered in multivariate analysis; (VII) long enough for outcomes to occur; (VIII) bias and limitations considered. Each category had 1 point, and then an overall maximum theoretical score was 8 points. Finally, the scores were expressed as numbers from 0 to 8, and better methodological quality was provided with higher values (Table S1).
Full table
Definition of outcomes
The primary outcomes were the OS, PFS, DFS or clinicopathological characteristics, including disease control rate (DCR), objective response rate (ORR), tumor stage, lymph node metastasis, distant metastasis, stage, differentiation, Eastern Cooperative Oncology Group (ECOG), histology and tumor size. Then, we conducted stratified analysis by histology type, stage, region of study and cut-off value for plasma fibrinogen. The effective value of OS, PFS and DFS was determined by the combination of HR and 95% confidence interval (CI). Estimated value was conducted indirectly from Kaplan-Meier plot using the methods reported by Tierney et al. (19) if a direct report of HR and corresponding 95% CI was not available. To assess the reliability, we calculated pooled HRs in studies with multivariate analysis. We employed relative risk (RR), odds ratio (OR) and standardized mean difference (SMD) to evaluate clinicopathological characteristics, including DCR, ORR, tumor stage, lymph node metastasis, distant metastasis, stage, differentiation, ECOG and histology.
Statistical analysis
Significant heterogeneity was identified if I2 more than 50% and P<0.05 with the Cochran’s Q-test and I2, then the random-effects model was used. Otherwise, it was calculated by the fixed-effects model without significant heterogeneity. The combination of the estimated risk was calculated by the Z test, and P<0.05 was considered statistically. Publication bias, assessed by rank correlation and linear regression method, was adjusted by the trim and fill method if publication bias existed (P<0.05). The R Statistical Software (version 3.5.1) was performed for statistical analyses. The “meta” package was applied to generate the pooled estimates and publication bias assessment. The “forestplot” package was used to produce forest plot. All P values were two sided.
Results
Trial flow
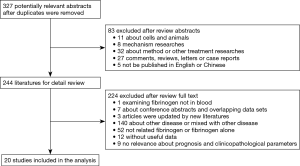
The result of the study searching process depicts in Figure 1. A total of 327 potentially relevant articles were identified, and 83 of them were excluded for the following reasons: 11 cell lines or animals, 8 mechanism researches, 32 method or other treatment researches, 27 comments, reviews, letters or case reports and 5 published in non-English and non-Chinese language. After reviewing full-text carefully, 20 articles were included in the analysis.
Study characteristics
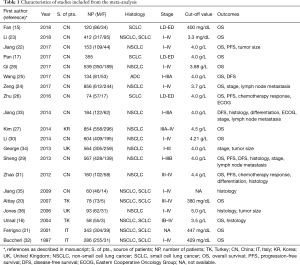
From 1997 to 2018, a total of 6,494 patients suffering from lung cancer were enrolled into the meta-analysis, the median sample size was 235 (ranged from 58 to 856). In this analysis, cut-off value of plasma fibrinogen was 4.0 g/L in eight studies. Besides, 16 (15-17,20-32), four (22,26,29,31) and 3 studies (25,29,33) were estimated by HR and corresponding 95% CI for OS, PFS and DFS, respectively. Two studies were evaluated by OR for histology (29,33), differentiation (29,33), chemotherapy response (DCR and ORR) (26,31) and performance status (26,33) and four (24,29,33,34) and three articles (24,29,33) for stage and lymph node metastasis, respectively. Furthermore, SMD was applied to appraise the effect of fibrinogen on clinicopathological characteristics, in which four studies (16,31,35,36) for histology and three for distant metastasis (16,24,35), tumor stage (24,35,36) and two for stage (24,35) (Table 1). Fourteen studies (15,20-32) investigated elevated plasma fibrinogen as an indicator of poor OS, and the other two studies (16,17) showed no significant impact on OS. Moreover, three studies (22,26,29) indicated that elevated plasma fibrinogen as a marker for poor PFS, and only one study (31) showed no effect on PFS.
Full table
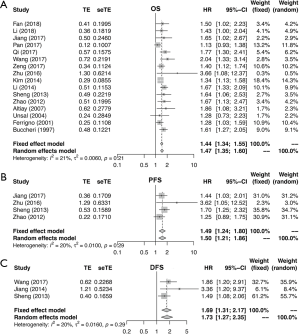
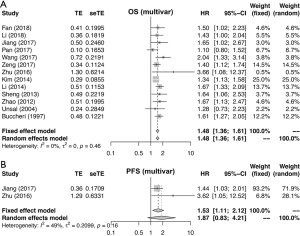
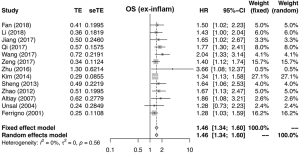
Meta-analysis of OS, PFS and DFS
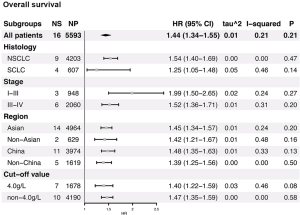
Figures 2 and S1-S3 shows results of each meta-analysis. The overall studies were systematic analyzed, with the combined HR 1.44 (95% CI =1.34–1.55), 1.49 (95% CI =1.24–1.80) and 1.69 (95% CI =1.31–2.17) for OS (Figure 2A), PFS (Figure 2B) and DFS (Figure 2C), respectively. In addition, the association was also validated in studies with multivariable analyses (HR=1.48, 95% CI =1.36–1.61 for OS, Figure S1A; HR=1.53, 95% CI =1.11–2.12 for PFS, Figure S1B). The results were consistent with analysis in overall studies. The pooled HR was 1.46 (95% CI =1.34–1.60) in studies excluded inflammatory disease (Figure S2). As shown in Figure 3, the results implied that elevated plasma fibrinogen level effected on poor OS in subgroup analyses about histology type, stage, region of study and cut-off value for plasma fibrinogen. The combined HR was 1.54 (95% CI =1.40–1.69) for NSCLC, 1.25 (95% CI =1.05–1.48) for SCLC, 1.99 (95% CI =1.50–2.65) for stage I–III, 1.52 (95% CI =1.36–1.71) for stage III–IV, 1.45 (95% CI =1.34–1.57), 1.42 (95% CI =1.21–1.67), 1.48 (95% CI =1.35–1.63), 1.39 (95% CI =1.25–1.56) for Asian, non-Asian, China, non-China, respectively. The pooled HRs were 1.40 (95% CI =1.22–1.59), 1.47 (95% CI =1.35–1.59) for studies with cut-off value equal to 4 g/L and was not 4 g/L.
Meta-analysis of clinicopathological characteristics
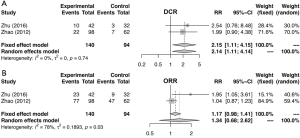
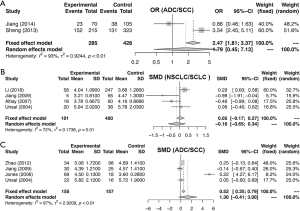
Similarly, the meta-analysis indicated that fibrinogen associated with clinicopathological characteristics. The pooled RRs and corresponding 95% CI were 2.15 (95% CI, 1.11–4.15) and 1.34 (95% CI, 0.68–2.62) for DCR (Figure S3A) and ORR (Figure S3B), respectively. In meta-analysis about TNM stage, the combined ORs were 1.50 (95% CI, 1.23–1.84) and 2.01 (95% CI, 1.66–2.44) lymph node metastasis (Figure 4A) and stage III–IV vs. stage I–II (Figure 4B), respectively. Moreover, the SMDs were 0.93 (95% CI, −0.42 to 2.29), 0.20 (95% CI, 0.04–0.36) and 0.31 (95% CI, 0.18–0.44) for tumor stage (Figure 4C), distant metastasis vs. no distant metastasis (Figure 4D) and stage III–IV vs. stage I–II (Figure 4E). No significance was found in poor differentiation (Figure S4A), and performance status (Figure S4B). In addition, the results showed no differences in histology (NSCLC and SCLC, adenocarcinoma and squamous cell carcinoma) (Figure S5A,B,C). The P value directly combined implied that plasma fibrinogen might affect tumor size (P=2.82×10−5).
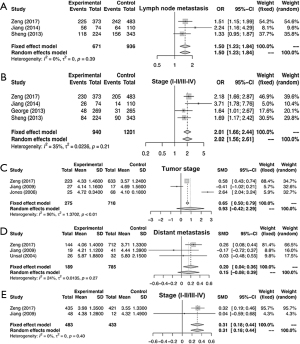
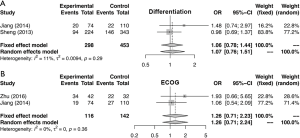
Test of heterogeneity
We analyzed the heterogeneity in all included studies between fibrinogen and the prognosis of lung cancer. The heterogeneity, sixteen studies for OS, four studies for PFS, three studies for DFS, were evaluated in a fixed-effects model. Moreover, the heterogeneity of included studies was 21%, 20% and 20% with the I2 value, respectively. Heterogeneity was no significance in the OS subgroup analysis (Figure 2A,B,C).
Publication bias
As shown in Figures S6,S7, the rank correlation and linear regression method were used to evaluate publication bias of the meta-analysis. We found a significant funnel plot asymmetry, with P=0.011 in the linear regression test and P=0.072 in the rank correlation test, demonstrating the existence of publication bias in OS. However, in PFS and DFS, no significant funnel plot asymmetry was observed, with P=0.323 and P=0.125 in the linear regression test, P=1.000 and P=0.117 in the rank correlation test. Furthermore, we used a trim-and-fill method to adjust the bias, which was developed by Duval and Tweedie (37). The adjusted result was similar with our results (HR =1.39, 95% CI, 1.30–1.49) in OS (Figure S7).
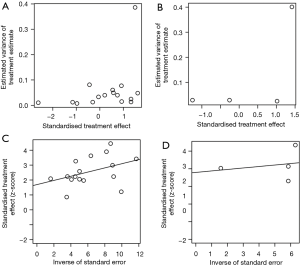
Discussion
Elevated pretreatment plasma fibrinogen predicted poor prognosis by meta-analysis in epithelial ovarian, digestive cancer (13,14). However, it was inconsistent whether fibrinogen was a prognosis factor in lung cancer. To our knowledge, this is the first systemic analysis about the association between fibrinogen and lung cancer prognosis. We performed a comprehensive analysis including 6,494 patients. It was showed that elevated pretreatment plasma fibrinogen levels could predict poor survival of lung cancer. Subgroup analysis also validated the result, according to region of study, histology type, cut-off value for plasma fibrinogen and HR estimated method for survival. Therefore, fibrinogen could play as a predictive factor of OS, PFS and DFS.
In addition, we also analyzed the association between fibrinogen and clinicopathological characteristics. We observed that elevated plasma fibrinogen affected TNM stage excluding tumor stage due to significant heterogeneity in lung cancer patients. However, plasma fibrinogen did not associate with differentiation and performance status and histology neither ADC vs. SCC nor NSCLC vs. SCLC. Due to limited sample size, the results of DCR and ORR were inconsistent. Considering the smoking was as a risk factor in lung cancer (38), we further found that smoking may affect plasma fibrinogen level and the pooled OR was 2.06 (95% CI, 1.64–2.59) analyzed in two studies (24,29).
In terms of fibrinogen in the prognosis of lung cancer, the evidence supported our conclusion as follow. Stroma-derived Fibrinogen-like Protein 2 promote tumor growth by activating cancer-associated fibroblasts in the tumor microenvironment in lung cancer (39). Meanwhile, the study revealed that fibrinogen levels are significantly high among different pathologic types of lung cancer patients (40). In addition, elevated fibrinogen was associated with performance status after lung cancer resection surgery (41). Moreover, high plasma fibrinogen was associated with poorer lymph node status, recurrence and advanced pathologic stage (33). Due to the close relationships between fibrinogen and worse clinical pathological features of lung cancer, the relation between fibrinogen and poor survival of lung cancer was understandable. Plasma fibrinogen level of lung cancer patients reduced obviously after surgery indicated the higher tumor burden the elevated plasma fibrinogen (33). Therefore, the plasma fibrinogen in pretreatment lung cancer may affect prognosis and metastasis by tumor microenvironment.
Though our study provided a relatively convincing conclusion that fibrinogen could play as a prognosis biomarker of lung cancer, certain inevitable limitations should be stated: (I) we did not exclude the study that reported the relation between serum fibrinogen and lung cancer prognosis. Fibrinogen do not exist in serum and we recognized that author was clerical error; (II) studies was excluded due to unavailable data for meta-analysis which may lead to publication bias (35,42,43). Moreover, this meta-analysis indicated existence of publication bias, however, we searched conference abstracts and the heterogeneity test and the trim and fill method demonstrated the conclusion was stable; (III) the therapeutic regimen was different may lead to bias. So, conducting high-quality research is necessary.
Overall, our meta-analysis indicated that pretreatment may as a poor prognosis factor in lung cancer. Moreover, plasma fibrinogen associated with TNM stage rather than ORR. Nonetheless, a well-designed prospective study with a large sample to confirm our results and cover the above limitations is essential.
Acknowledgments
Funding: This study was supported by National Natural Science Foundation of China (81472782); the National Key R&D Program of China (2017YFC0908200).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Torre LA, Siegel RL, Ward EM, et al. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev 2016;25:16-27. [Crossref] [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Mantovani G, Madeddu C, Gramignano G, et al. Subcutaneous interleukin-2 in combination with medroxyprogesterone acetate and antioxidants in advanced cancer responders to previous chemotherapy: phase II study evaluating clinical, quality of life, and laboratory parameters. J Exp Ther Oncol 2003;3:205-19. [Crossref] [PubMed]
- Paesmans M, Sculier JP, Libert P, et al. Prognostic factors for survival in advanced non-small-cell lung cancer: univariate and multivariate analyses including recursive partitioning and amalgamation algorithms in 1,052 patients. The European Lung Cancer Working Party. J Clin Oncol 1995;13:1221-30. [Crossref] [PubMed]
- Albain KS, Crowley JJ, LeBlanc M, et al. Determinants of improved outcome in small-cell lung cancer: an analysis of the 2,580-patient Southwest Oncology Group data base. J Clin Oncol 1990;8:1563-74. [Crossref] [PubMed]
- Foster NR, Mandrekar SJ, Schild SE, et al. Prognostic factors differ by tumor stage for small cell lung cancer: a pooled analysis of North Central Cancer Treatment Group trials. Cancer 2009;115:2721-31. [Crossref] [PubMed]
- Finkelstein DM, Ettinger DS, Ruckdeschel JC. Long-term survivors in metastatic non-small-cell lung cancer: an Eastern Cooperative Oncology Group Study. J Clin Oncol 1986;4:702-9. [Crossref] [PubMed]
- Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol 2005;23:5900-9. [Crossref] [PubMed]
- Tennent GA, Brennan SO, Stangou AJ, et al. Human plasma fibrinogen is synthesized in the liver. Blood 2007;109:1971-4. [Crossref] [PubMed]
- Repetto O, De Re V. Coagulation and fibrinolysis in gastric cancer. Ann N Y Acad Sci 2017;1404:27-48. [Crossref] [PubMed]
- Allin KH, Bojesen SE, Nordestgaard BG. Inflammatory biomarkers and risk of cancer in 84,000 individuals from the general population. Int J Cancer 2016;139:1493-500. [Crossref] [PubMed]
- Staton CA, Brown NJ, Lewis CE. The role of fibrinogen and related fragments in tumour angiogenesis and metastasis. Expert Opin Biol Ther 2003;3:1105-20. [Crossref] [PubMed]
- Luo Y, Kim HS, Kim M, et al. Elevated plasma fibrinogen levels and prognosis of epithelial ovarian cancer: a cohort study and meta-analysis. J Gynecol Oncol 2017;28:e36. [Crossref] [PubMed]
- Lin Y, Liu Z, Qiu Y, et al. Clinical significance of plasma D-dimer and fibrinogen in digestive cancer: A systematic review and meta-analysis. Eur J Surg Oncol 2018;44:1494-503. [Crossref] [PubMed]
- Fan S, Guan Y, Zhao G, et al. Association between plasma fibrinogen and survival in patients with small-cell lung carcinoma. Thorac Cancer 2018;9:146-51. [Crossref] [PubMed]
- Unsal E, Atalay F, Atikcan S, et al. Prognostic significance of hemostatic parameters in patients with lung cancer. Respir Med 2004;98:93-8. [Crossref] [PubMed]
- Pan H, Shi X, Xiao D, et al. Nomogram prediction for the survival of the patients with small cell lung cancer. J Thorac Dis 2017;9:507-18. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495-9. [Crossref] [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- Altiay G, Ciftci A, Demir M, et al. High Plasma d-dimer Level is Associated with Decreased Survival in Patients with Lung Cancer. Clin Oncol (R Coll Radiol) 2007;19:494-8. [Crossref] [PubMed]
- Ferrigno D, Buccheri G, Ricca I. Prognostic significance of blood coagulation tests in lung cancer. Eur Respir J 2001;17:667-73. [Crossref] [PubMed]
- Jiang LY, Zhang XF, Ding FB, et al. Prognostic implications of plasma fibrinogen and serum C-reactive protein levels in non-small cell lung cancer resection and survival. Trop J Pharm Res 2017;16:665-72. [Crossref]
- Li SQ, Jiang YH, Lin J, et al. Albumin-to-fibrinogen ratio as a promising biomarker to predict clinical outcome of non-small cell lung cancer individuals. Cancer Med 2018;7:1221-31. [Crossref] [PubMed]
- Zeng Q, Xue N, Dai D, et al. A Nomogram based on Inflammatory Factors C-Reactive Protein and Fibrinogen to Predict the Prognostic Value in Patients with Resected Non-Small Cell Lung Cancer. J Cancer 2017;8:744-53. [Crossref] [PubMed]
- Wang YQ, Zhi QJ, Wang XY, et al. Prognostic value of combined platelet, fibrinogen, neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in patients with lung adenosquamous cancer. Oncol Lett 2017;14:4331-8. [Crossref] [PubMed]
- Zhu LR, Li J, Chen P, et al. Clinical significance of plasma fibrinogen and D-dimer in predicting the chemotherapy efficacy and prognosis for small cell lung cancer patients. Clin Transl Oncol 2016;18:178-88. [Crossref] [PubMed]
- Kim KH. Prognostic significance of initial platelet counts and fibrinogen level in advanced non-small cell lung cancer. J Korean Med Sci 2014;29:507-11. [Crossref] [PubMed]
- Qi Y, Fu J. Research on the coagulation function changes in non small cell lung cancer patients and analysis of their correlation with metastasis and survival. J BUON 2017;22:462-7. [PubMed]
- Sheng L, Luo M, Sun X, et al. Serum fibrinogen is an independent prognostic factor in operable nonsmall cell lung cancer. Int J Cancer 2013;133:2720-5. [PubMed]
- Li Y, Wei S, Wang J, et al. Analysis of the factors associated with abnormal coagulation and prognosis in patients with non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 2014;17:789-96. [PubMed]
- Zhao J, Zhao M, Jin B, et al. Tumor response and survival in patients with advanced non-small-cell lung cancer: the predictive value of chemotherapy-induced changes in fibrinogen. BMC Cancer 2012;12:330. [Crossref] [PubMed]
- Buccheri G, Ferrigno D, Ginardi C, et al. Haemostatic abnormalities in lung cancer: prognostic implications. Eur J Cancer 1997;33:50-5. [Crossref] [PubMed]
- Jiang HG, Li J, Shi SB, et al. Value of fibrinogen and D-dimer in predicting recurrence and metastasis after radical surgery for non-small cell lung cancer. Med Oncol 2014;31:22. [Crossref] [PubMed]
- George RS, Smith MO, Sharkey AJ, et al. Is plasma fibrinogen a novel independent prognostic factor in patients undergoing surgery for non-small cell lung cancer? Lung Cancer 2013;79:S27. [Crossref]
- Jiang Z, Sang H, Ge H, et al. The study of prethrombotic state in patients with lung cancer. Zhongguo Fei Ai Za Zhi 2009;12:44-8. [PubMed]
- Jones JM, McGonigle NC, McAnespie M, et al. Plasma fibrinogen and serum C-reactive protein are associated with non-small cell lung cancer. Lung Cancer 2006;53:97-101. [Crossref] [PubMed]
- Duval S, Tweedie R.. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455-63. [Crossref] [PubMed]
- Hong S, Mok Y, Jeon C, et al. Tuberculosis, smoking and risk for lung cancer incidence and mortality. Int J Cancer 2016;139:2447-55. [Crossref] [PubMed]
- Zhu Y, Zhang L, Zha H, et al. Stroma-derived Fibrinogen-like Protein 2 Activates Cancer-associated Fibroblasts to Promote Tumor Growth in Lung Cancer. Int J Biol Sci 2017;13:804-14. [Crossref] [PubMed]
- Nakano K, Sugiyama K, Satoh H, et al. Risk factors for disseminated intravascular coagulation in patients with lung cancer. Thorac Cancer 2018;9:931-8. [Crossref] [PubMed]
- Araujo AS, Nogueira IC, Gomes Neto A, et al. The impact of lung cancer resection surgery on fibrinogen and C-reactive protein and their relationship with patients outcomes: A prospective follow up study. Cancer Biomark 2016;16:47-53. [Crossref] [PubMed]
- Tas F, Kilic L, Serilmez M, et al. Clinical and prognostic significance of coagulation assays in lung cancer. Respir Med 2013;107:451-7. [Crossref] [PubMed]
- George R, Smith M, Sharkey A, et al. Is plasma fibrinogen a novel independent prognostic factor in patients undergoing surgery for non-small cell lung cancer? Interact Cardiovasc Thorac Surg 2013;79:S27.