Preoperative hypoxemia in patients with type A acute aortic dissection: a retrospective study on incidence, related factors and clinical significance
Introduction
About 2.6 to 3.5/105 individuals develop acute type A aortic dissection (AAD) every year. Affected patients experience acute onset and rapid progression necessitating emergency surgical treatment (1). The in hospital mortality rate in aortic dissection patients is 34% in a recent study (2). AAD is often accompanied by several severe systemic pathophysiological changes, of which hypoxemia (HO) is the most common. Patients with HO have an oxygen index (PaO2/FiO2, OI) ≤300 mmHg resulting from damage to alveolar epithelial and capillary endothelial cells (3). Patients with AAD and preoperative HO are at increased risk for postoperative acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Affected patients have prolonged intubation times and/or increased mortality rates after surgery (4,5).
We previously reported our findings in a small group of AAD patients. Among them, 53.8% of AAD patients had ALI at initial presentation, and elevated levels of several inflammatory cytokines were found in these patients (6). While risk factors for poor outcome in AAD patients have been identified, the role of preoperative HO in determining postoperative HO has not been evaluated. We now report the findings in a larger group of patients, with greater power to detect factors associated with HO, including preoperative HO.
Methods
Approval by the Institutional Research Ethics Board of Beijing Anzhen Hospital was obtained to conduct this prospective, single-center, observational study (No. 2013027). Individual patient consent was waived by the Research Ethics Board due to the observational nature of the study.
Study design
This retrospective study reviewed the charts of all AAD patients who underwent surgical treatment in Beijing Anzhen Hospital, China, between January 2015 and February 2018.
Patients
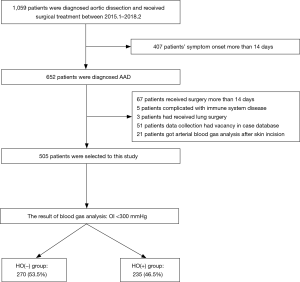
All patients diagnosed with AAD were included in this study. Inclusion criteria were patients: (I) diagnosed with AAD confirmed by CT angiography of aorta that (II) underwent surgical treatment. Exclusion criteria were (I) no arterial blood gas analysis (ABG) performed before the initial skin incision; (II) immune system disease, chronic obstructive pulmonary disease, chronic renal failure, end-stage liver disease, connective tissue diseases, or malignant disease; and (III) missing data in the case database (Figure 1).
Grouping
The HO(+) group of patients had a preoperative OI <300 mmHg on ABG. Patients with ABG OI greater than 300 mmHg formed the HO(−) group.
Data collection
Information regarding patient gender, age, history of hypertension (yes/no), type 2 diabetes (yes/no), body mass index (BMI), pleural effusion (yes/no), pericardial effusion (yes/no), time since surgery, coagulation tests, and ABG analysis were collected from the case database.
OI collection
After intubation, mechanical ventilation with a FiO2 of 100% oxygen and 5 cmH2O positive end-expiratory pressure was administered for 10 minutes. End-tidal carbon dioxide pressure was maintained at 35 to 45 mmHg by adjusting tidal volume to 6 to 8 mL/kg and respiratory rate to 10 to 15 min−1. OI was then measured by ABG analysis.
Serologic examination
Routine blood tests and biochemical, markers of myocardial damage were monitored for 24 hours before surgery. The results obtained closest to surgery were recorded for this study.
Anesthetic management
Patient vital signs were regularly monitored after hospital admission. Systolic blood pressure was maintained at <120 mmHg (1 mmHg =0.133 kPa). All patients received standard anaesthesia including 10 mg morphine i.v. before entering the operating room, and 5-lead ECG, pulse oximetry (SpO2), blood pressure, central venous pressure (CVP) and bispectral index (BIS) monitoring. Anesthesia was induced using midazolam (3 to 5 mg), etomidate (150 to 300 µg/kg), sufentanil (1 to 3 µg/kg), and cis-atracurium (0.2 mg/kg) for muscle relaxation. Patient hemodynamics including systolic pressure, CVP and heart rate were maintained and fluid replenished as needed during induction. Sufentanil (0.5 to 1.5 µg/k), propofol (2 to 5 mg/kg /h) and cis-atracurium (0.11 mg/kg) were administered to maintain a BIS value of 45–55%.
Primary outcome and secondary outcome
Our primary outcome was the incidence of preoperative hypoxemia in patients undergoing surgery for AAD. Our secondary outcomes included measurement of factors related to preoperative HO, postoperative mortality, extubation time, ICU duration, and activity of daily living scale score.
Statistical analysis
SPSS Statistics Desktop (version 21.0.0 for Mac OS, IBM, Armonk, NY, USA) was used for statistical analysis. Mean ± standard deviation was used to express continuous data, and frequencies were used to express categorical data. Normally distributed continuous variables were compared using a two-tailed Student’s t-test. Wilcoxon rank sum testing was used for inter-group comparisons when parametric data were not normally distributed. χ2 testing was performed to compare categorical variables. Multivariable binary logistic regression analysis was used to identify independent prognostic factors. A P value less than 0.05 indicated a significant difference. Receiver operating characteristic (ROC) curves were used to determine the power of the multivariable logistic regression model for HO. The area under the ROC curve (AUROC) was calculated to assess the discriminative ability of the identified factors for HO. The sample size was calculated using a 1-sample, 1-sided test with a power of 0.99 and α<0.05. Formulas used are shown in Figure S1 and Table S1.

Full table
Results
Baseline characteristics
A total of 1,059 patients were diagnosed with aortic dissection (AD) during the study period. Four hundred and seven of these patients had symptoms for more than 14 days, leaving 652 with AAD. A total of 147 additional patients were excluded (67 received surgery more than 14 days prior to evaluation, 5 had a compromised immune system disease, 3 also underwent lung surgery, 51 had missing data, and 21 had their first ABG analysis after the start of surgery). Five hundred and five cases were evaluable for this study (Figure 1).
The result of primary outcome and secondary outcome
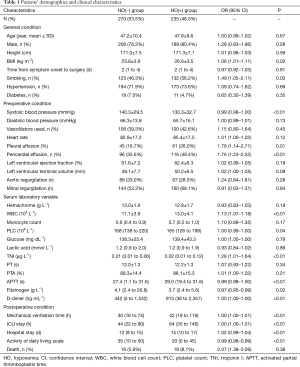
Two hundred and thirty-five (46.5%) patients were HO(+). One hundred and eighty-nine (79.1%) were male, and their average age was 47.8 years. Their average OI was 371.8±57.5 mmHg. The average OI of the HO(−) group was 230.2±47.1 mmHg. The duration of postoperative mechanical ventilation, ICU stay and hospital stay was all longer in HO(+) patients, compared to that of HO(−) patients (P<0.01 for all comparisons) (Table 1). The HO(−) group had higher activity of daily living scale scores than the HO(+) group (P<0.01; Table 1).
Full table
The result of Univariate analysis and Multivariable binary logistic regression
BMI, systolic blood pressure, smoking, pleural effusion, pericardial effusion, platelet count (PLC), white blood cell count (WBC), troponin I (TNI), activated partial thromboplastin time (APTT), fibrinogen, and D-dimer levels were associated with the presence of preoperative HO (Table 1).
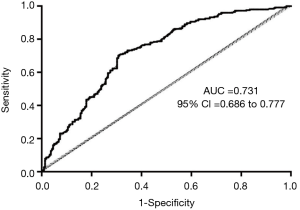
Multivariable binary logistic regression identified fibrinogen (OR, 0.972; 95% CI, 0.953–0.991; P<0.01), WBC (OR, 1.131; 95% CI, 1.068–1.197; P<0.01), systolic blood pressure (OR, 0.992; 95% CI, 0.985–0.998; P=0.017), smoking history (OR, 1.542; 95% CI, 1.025–2.319; P=0.038), and pleural effusion (OR, 2.103; 95% CI, 1.267–3.492; P<0.01) as related factors for HO (Table 2). The AUROC of this multivariable binary logistic regression analysis was 0.731 (95% CI, 0.686–0.777) (Figure 2).

Full table
The relationship between OI and the duration of symptoms
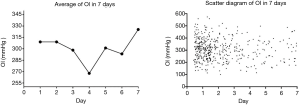
OI was negatively related to the duration of symptoms (R2=0.01; P=0.02) in AAD patients with an onset of symptoms less than 4 days before surgery. The average condition of OI on the fourth day (258.1±86.7 mmHg) was significantly lower than it on the first day (307.5±88.0 mmHg, P<0.01) in these patients (Figure 3).
Discussion
AAD is the most frequent and catastrophic manifestation of aortic syndrome with a high associated morbidity and mortality (7). Preoperative ALI occurred in 46.5% (235/505) of AAD treated patients similar to other studies [Xudong, 53.8% (6); Sugano 51.0% (8)].
AAD can occur after a medial intima tear and formation of a false lumen. This collection of blood in the aortic wall contributes to an inflammatory response (9). The progression of local AAD related inflammation can trigger systemic inflammation and adversely affect multiple organs (10-12). Pathologic findings observed with activation of the inflammatory system are similar to those found in our AAD patients. Secondary activation of the thromboplastin system results in consumptive coagulopathy (13). Activation of the fibrinolytic system degrades fibrinogen into fibrin with thrombus and microemboli formation. Microemboli obstructs pulmonary capillaries and affect lung ventilation. Patients with aortic dissection often have increased D-dimer levels, leading to further lung injury (14). Pro-inflammatory factors such as TXA2 can be released with the development of coagulopathy (14). Patients treated had a negative correlation between platelet count and TXA2 levels (r=−0.22, P=0.02), compatible with the described related pathological findings. Serum D-dimer levels were significantly higher in the HO(+) group, compared to the HO(−) group, similar to the findings of Vieira (15). Together, these findings contribute to the abnormalities found in HO(+) patients with AAD.
Vascular inflammation like that described in AAD is associated with increased local levels of vascular adhesion molecules, macrophage-activating cytokines, and chemotactic factors. Increased secretion of inflammatory factors in the lung leads to pulmonary capillary endothelium edema, an abnormal ventilation-perfusion ratio, intrapulmonary shunting, and decreased pulmonary gas exchange (16). These changes lead to alveolar fluid accumulation, lung injury, and pleural effusion (17).
Preoperative HO is a related factor for postoperative ARDS (18,19). Patients we examined had a correlation between WBC, fibrinogen levels, SBP, smoking history, and pleural effusion, and the presence of preoperative HO. Our patient’s recovery, measured by time to extubation, and length of ICU and hospital stay, was significantly longer in patients with HO. These patients also had a lower activity of daily living scale score, compared to HO(−) patients. Together, these findings support the previously reported association between HO and inflammation, ischemia, and reperfusion injury of the lung (19).
The secretion of inflammatory cytokines mirrors the development of AAD symptoms and OI deterioration. C-reactive protein, an indicator of inflammatory response, peaks in expression on the third day of symptoms of AAD and IL-6 (20), another inflammatory cytokine, peaks in expression on the fifth day of symptoms of AAD. Patients we treated had a significant downward trend of OI during the first 4 days of the onset of symptoms of AAD, with the lowest levels on the fourth day. An upward trend in OI was seen after the fourth day before surgery.
Several clinical findings were associated with the development of HO. Increasing patient age and obesity was associated with ALI in the AAD patients we treated. Respiratory function is known to decline with increasing age. Obesity is more frequently associated with hypoxemia, particularly when hypoventilation syndrome and obstructive sleep apnea are present (21,22). These findings may have contributed to ALI in our patients.
Inadequately controlled hypertension is commonly found in patients with AAD (23). Hypertension can cause atherosclerosis, adversely affecting pulmonary circulation. AAD patients we treated with lower systolic blood pressure were more likely to have low OI levels. These findings could result from altered pulmonary circulation and insufficient tissue perfusion related to low blood pressure.
There were several limitations to this study. This was a retrospective study that needs independent validation. Patients evaluated here all underwent surgery. The findings in similar patients who did not undergo surgery were unknown. OI was calculated based on the respiratory parameters of intubated patients. This finding may not be accurate in patients with underlying lung disease not related to AAD.
For the protection of preoperative lung injury in patients with AAD, we suggest that patients prone to AAD should give up smoking and control their weight as soon as possible. For patients who have been diagnosed with AAD, preoperative intravenous injection of ustatin and inhalation of xenon and other anti-inflammatory drugs can inhibit the inflammatory response (24). In addition, monitoring and management of AAD patients with HO should be strengthened.
In conclusion, about 46.5% of the AAD patients we treated had HO. Their recovery from surgery was slower than that of patients without HO. Altered fibrinogen, WBC, systolic blood pressure levels, positive smoking history, and a pleural effusion were associated with the presence of preoperative HO. Prospective studies are needed to validate these findings.
Acknowledgments
Assistance with the article: we thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding: This study was supported by grants from the Beijing Municipal Science & Technology Commission (Z161100000513067, and Z171100001017083). Sponsors had no influence on the design, analysis, or publication of this study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Approval by the Institutional Research Ethics Board of Beijing Anzhen Hospital was obtained to conduct this prospective, single-center, observational study (No. 2013027). Individual patient consent was waived by the Research Ethics Board due to the observational nature of the study.
References
- Kurabayashi M, Okishige K, Azegami K, et al. Reduction of the PaO2/FiO2 ratio in acute aortic dissection - relationship between the extent of dissection and inflammation. Circ J 2010;74:2066-73. [Crossref] [PubMed]
- Mkalaluh S, Szczechowicz M, Mashhour A, et al. Total aortic arch replacement using elephant trunk or frozen elephant trunk technique: a case-control matching study. J Thorac Dis 2018;10:6192-200. [Crossref] [PubMed]
- Johnson ER, Matthay MA. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv 2010;23:243-52. [Crossref] [PubMed]
- Wong DR, Lemaire SA, Coselli JS. Managing dissections of the thoracic aorta. Am Surg 2008;74:364-80. [PubMed]
- Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol 2011;6:147-63. [Crossref] [PubMed]
- Pan X, Lu J, Cheng W, et al. Independent factors related to preoperative acute lung injury in 130 adults undergoing Stanford type-A acute aortic dissection surgery: a single-center cross-sectional clinical study. J Thorac Dis 2018;10:4413-23. [Crossref] [PubMed]
- Sheikh AS, Ali K, Mazhar S. Acute aortic syndrome. Circulation 2013;128:1122-7. [Crossref] [PubMed]
- Sugano Y, Anzai T, Yoshikawa T, et al. Serum C-reactive protein elevation predicts poor clinical outcome in patients with distal type acute aortic dissection: association with the occurrence of oxygenation impairment. Int J Cardiol 2005;102:39-45. [Crossref] [PubMed]
- Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation 2005;111:816-28. [Crossref] [PubMed]
- Ma WG, Zheng J, Dong SB, et al. Sun's procedure of total arch replacement using a tetrafurcated graft with stented elephant trunk implantation: analysis of early outcome in 398 patients with acute type A aortic dissection. Ann Cardiothorac Surg 2013;2:621-8. [PubMed]
- Sun L, Qi R, Zhu J, et al. Repair of acute type A dissection: our experiences and results. Ann Thorac Surg 2011;91:1147-52. [Crossref] [PubMed]
- del Porto F, Proietta M, Tritapepe L, et al. Inflammation and immune response in acute aortic dissection. Ann Med 2010;42:622-9. [Crossref] [PubMed]
- Guan XL, Wang XL, Liu YY, et al. Changes in the Hemostatic System of Patients With Acute Aortic Dissection Undergoing Aortic Arch Surgery. Ann Thorac Surg 2016;101:945-51. [Crossref] [PubMed]
- Turak O, Canpolat U, Ozcan F, et al. D-dimer level predicts in-hospital mortality in patients with infective endocarditis: a prospective single-centre study. Thromb Res 2014;134:587-92. [Crossref] [PubMed]
- Vieira-de-Abreu A, Campbell RA, Weyrich AS, et al. Platelets: versatile effector cells in hemostasis, inflammation, and the immune continuum. Semin Immunopathol 2012;34:5-30. [Crossref] [PubMed]
- Sarkar M, Niranjan N, Banyal PK. Mechanisms of hypoxemia. Lung India 2017;34:47-60. [Crossref] [PubMed]
- Piantadosi CA, Schwartz DA. The acute respiratory distress syndrome. Ann Intern Med 2004;141:460-70. [Crossref] [PubMed]
- Talla K, Dmytriw AA, Nguyen E. Acute type A aortic dissection with mediastinal hematoma mimicking massive pulmonary embolus. Int J Cardiovasc Imaging 2017;33:259-60. [Crossref] [PubMed]
- Wu Z, Dai F, Ren W, et al. Angiotensin II induces apoptosis of human pulmonary microvascular endothelial cells in acute aortic dissection complicated with lung injury patients through modulating the expression of monocyte chemoattractant protein-1. Am J Transl Res 2016;8:28-36. [PubMed]
- Wen D, Zhou XL, Li JJ, et al. Biomarkers in aortic dissection. Clin Chim Acta 2011;412:688-95. [Crossref] [PubMed]
- Lumachi F, Marzano B, Fanti G, et al. Relationship between body mass index, age and hypoxemia in patients with extremely severe obesity undergoing bariatric surgery. In Vivo 2010;24:775-7. [PubMed]
- Gong MN, Thompson BT, Williams P, et al. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med 2005;33:1191-8. [Crossref] [PubMed]
- Neukamm AM, Hoiseth AD, Hagve TA, et al. High-sensitivity cardiac troponin T levels are increased in stable COPD. Heart 2013;99:382-7. [Crossref] [PubMed]
- Jin M, Yang Y, Pan X, et al. Effects of pulmonary static inflation with 50% xenon on oxygen impairment during cardiopulmonary bypass for stanford type A acute aortic dissection: A pilot study. Medicine (Baltimore) 2017;96:e6253. [Crossref] [PubMed]