
Associations between changes in oxygenation, dead space and driving pressure induced by the first prone position session and mortality in patients with acute respiratory distress syndrome
Introduction
One randomized clinical trial in patients with moderate to severe acute respiratory distress syndrome (ARDS) convincingly showed mortality benefit from turning patients from the supine to the prone position (PP) (1). Two meta-analyses confirmed the benefit of the PP (2,3), though the PP remains underutilized (4,5). Different mechanisms have been proposed to explain survival benefit induced by repositioning to the PP, including homogenizing of transpleural pressures, decreasing lung stress and strain by increasing lung volumes, and decreasing overdistension by redistribution of lung ventilation.
Outcome prediction in ARDS patients is challenging (6,7), especially in patients with severe refractory hypoxemia in whom PP sessions are often needed. In ARDS patients, the ratio of arterial oxygen partial pressure (PaO2) to fractional inspired oxygen (FiO2) ratio (PaO2/FiO2) (8), dead space fraction (VD/VT) (9,10), and respiratory system driving pressure (ΔPRS) (11,12) have an association with mortality. Turning a patient from supine to the PP affects PaO2/FiO2, VD/VT and ΔPRS through various mechanisms, and changes induced by this repositioning could be helpful in outcome prediction (6,7,13). A recent post hoc analysis of the randomized clinical trial (RCT) mentioned above (1) did not confirm this, though (14). It must be mentioned, however, that that analysis only used changes in PaO2/FiO2 for outcome prediction, and also that changes were calculated from blood gas analysis results after positioning in the PP and before repositioning to the supine position.
The aim of the current analysis, therefore, was to determine the association between PP-induced changes in PaO2/FiO2, as well as VD/VT and ΔPRS, using ventilation variables collected before placing patients in the PP and after repositioning to supine, and outcomes. For this purpose, the ‘Molecular Diagnosis and Risk stratification of Sepsis’ study (MARS), a large cohort study including patients with ARDS needing PP, was reanalyzed. The primary hypothesis tested was that changes in PaO2/FiO2, VD/VT and ΔPRS induced by the first PP session have prognostic capacity for mortality.
Methods
Design and ethical approval
MARS was a conveniently-sized observational study capturing granular data of intensive care unit (ICU) patients in two Dutch hospitals (15,16). In MARS, detailed demographic and clinical data, including ventilator settings and ventilation variables, as well as outcome data were prospectively collected from ICU admission and start of invasive ventilation, until extubation and ICU discharge, and 1-year mortality. The Institutional Review Board approved the study protocol of the parent study and the use of an opt-out consent procedure (protocol no. 10-056C). MARS was registered at www.clinicaltrials.gov (identifier NCT01905033). The current report adheres to the STROBE guidelines (17).
Patients
Patients were eligible for participation in MARS if they had an expected length of stay in the ICU of more than 24 hours. MARS itself used no exclusion criteria.
For the purpose of this post hoc analysis, the following additional inclusion criteria were used: (I) admitted to the ICU of the Amsterdam University Medical Centers, location Academic Medical Center (AMC), Amsterdam, The Netherlands; (II) having ARDS; and (III) use of the PP for refractory hypoxemia. The single reason for exclusion was impossibility to capture complete sets of variables, necessary for calculating PaO2/FiO2, VD/VT or ΔPRS before and after the first PP session. Thus, patients who died during the first PP session and patients who were transferred to another hospital while in the PP, were excluded.
ARDS diagnosis
The MARS team existed of extensively trained clinical researchers who scored all patients prospectively for presence of acute lung injury (ALI) or ARDS according to the American–European Consensus Conference criteria for ARDS (18). Patients were later re-classified using the Berlin Definition for ARDS that was introduced after initiation of the MARS (8). All patients initially diagnosed with ARDS also fulfilled the Berlin definition and could thus be re-classified as having mild, moderate or severe ARDS based on the lowest PaO2/FiO2 within the first 24 hours after the initial diagnosis of ARDS (8).
Calculation of PaO2/FiO2, VD/VT and ΔPRS
PaO2 was measured using a point-of-care blood gas analyzer (Rapidlab 1265, Siemens Healthcare GmbH, Kemnath, Germany). Continuous end-tidal CO2 (EtCO2) monitoring was performed using main-stream capnography (Philips, Best, The Netherlands). FiO2, positive end-expiratory pressure (PEEP) and maximum airway pressure (Pmax) were recorded from the ventilator at the moment of blood sampling for blood gas analyses.
PaO2/FiO2, VD/VT, and ΔPRS were calculated using variables obtained in the supine position before and after completion of the first PP session. For these calculations, data 1 hour before start, and 1 hour after repositioning to the supine position were used. If these data were missing, we used data closest to the 1-hour time point but never more than 3 hours before start and 3 hours after ending the first PP session. PaO2/FiO2 was calculated by dividing PaO2 by FiO2. VD/VT was calculated using the modified Bohr formula in which VD/VT = (PaCO2–PetCO2)/PaCO2 (7). ΔPRS was calculated by subtracting PEEP from Pmax, measured at zero flow.
Primary and secondary endpoints
The primary endpoint was the prognostic capacity of PaO2/FiO2, VD/VT and ΔPRS, expressed in the area under the Receiver Operating Characteristic (ROC) for all–cause mortality at day 28. Secondary outcomes were the prognostic capacities of PaO2/FiO2, VD/VT and ΔPRS for ICU and 1-year mortality. These outcomes should be seen as explorative, and therefore no correction for multiple testing was performed.
The local guideline for ventilatory support
The local guideline for ventilatory support advised on main ventilator settings in patients with ARDS, and indications for the PP (19). Briefly, ARDS patients were to receive invasive ventilation using a pressure-controlled ventilation mode, or pressure support ventilation mode, with tidal volume targeting 6 ml per kilogram of predicted body weight and PEEP following a ‘lower PEEP/FiO2 table’, where every increase in PEEP was to be preceded by a recruitment maneuver. Nurses and physicians adjusted the inspiratory pressure to maintain the correct tidal volume.
Patients were assessed at least three times daily to determine whether weaning could start, consisting of switches to a pressure support mode of ventilation, if not yet spontaneously breathing. If pressure support ventilation was accepted, the level of support was gradually decreased to a minimum of 5 cmH2O at least three times per day. Tracheal extubation was performed at the discretion of attending physicians, based on general extubation criteria (20).
The local guideline for prone positioning
The local guideline dictated that PP sessions were indicated in patients in whom the PaO2/FiO2 remained <125 mmHg despite increases in PEEP level ≥10 cmH2O at a minimum FiO2 ≥0.6. Sessions were repeated if PaO2/FiO2 remained or dropped to <125 mmHg at a PEEP of ≥10 cmH2O at a minimum FiO2 ≥0.6 when back in the supine position. Of note, the local guidelines for ventilation do not allow changes in PEEP, neither when a patient is in the PP nor before the results of blood gas analyses become available when a patient is returned to supine.
Power calculation
A formal power calculation was not performed, but instead this analysis used all patients who had received at least one PP session during the 3 years MARS enrolled patients.
Analysis plan
Variables and parameters were expressed as medians [25th to 75th interquartile range (IQR) for continuous variables, or percentages for categorical variables. Continuous variables and parameters were analyzed using a Mann-Whitney U-test or a Welch two-ample t-test, and proportions were compared using a Fisher exact test.
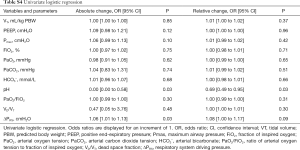
First, absolute and relative changes between PaO2/FiO2, VD/VT and ΔPRS before to after the first PP session were calculated and compared between 28-day survivors and non-survivors using the Mann-Whitney U-test. The association between PP-induced changes in PaO2/FiO2, VD/VT, and ΔPRS, and mortality was determined using a univariate and multivariable logistic regression. A propensity score was entered into the multivariate model as a covariate to correct for disease severity and other baseline factors that could have influenced patient outcome (21), using the following predefined baseline variables: disease severity [age, an age-corrected Acute Physiology and Chronic Health Evaluation (APACHE) IV score], and baseline PaO2/FiO2, VD/VT, ΔPRS, VT, and respiratory rate (RR). These variables were selected because they are all suggested to have an association with mortality. As there were 41 events of the outcome of interest, i.e., 28-day mortality, and the general rule of thumb is that 10 events of the outcome of interest are required for each variable entered in the model (22), the multivariable analysis using only 2 variables can be seen as sufficiently powered. An etiological model was chosen because of the restricted sample size.
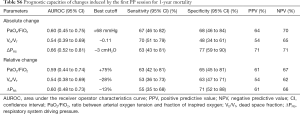
Second, area under the ROC curve for 28-day mortality was calculated for PP-induced changes in PaO2/FiO2, VD/VT and ΔPRS. The optimal cutoff was determined using the Youden index (23), and sensitivity, specificity, and positive and negative predictive values were calculated. A priori, an area under the curve (AUC) of ≤0.6 was considered ‘poor’, 0.6–0.7 ‘fair’, 0.7–0.8 ‘good’, 0.8–0.9 ‘very good’ and ≥0.9 ‘excellent’ (24).
Statistical analyses were performed using R and the R-studio interface (R version 3.0, www.r-project.org). A P value <0.05 was considered as statistically significant.
Results
Patients
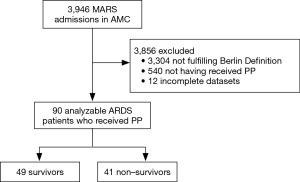
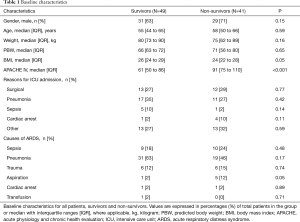
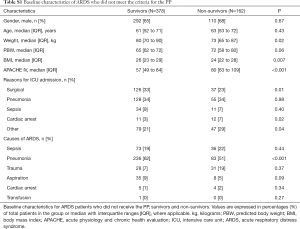
Of 3,946 patients enrolled in MARS in the participating center, 642 (16%) patients were diagnosed with ARDS (Figure 1). Of these, 102 (16%) patients were repositioned in the PP for at least one session. After exclusion of patients with incomplete datasets, 90 patients remained in the analysis. Baseline demographics, ventilation variables, and ventilations parameters before and after the first PP session are presented in Tables 1,2. All cause 28-day mortality rate was 46%. Patients who died were sicker according to the APACHE IV scores. Patients who died had higher VD/VT at baseline, while baseline PaO2/FiO2 and ΔPRS were not different between survivors and non-survivors. Patients with ARDS in MARS study who did not meet the criteria for and thus were not placed in the PP had similar baseline characteristics but were less sick according to the median APACHE IV score (Table S1). Time between start of invasive ventilation and the first PP session, total duration of the first PP session, and the total number of PP sessions during the entire ICU stay were not different between survivors and non-survivors (Table S2). In 90% and 96% of cases, data to calculate the parameters of interest were available within the last hour before the first PP session, and the first hour after repositioning to supine, respectively.
Full table
Full table
Full table

Full table
PP-induced changes in PaO2/FiO2, VD/VT, and ΔPRS
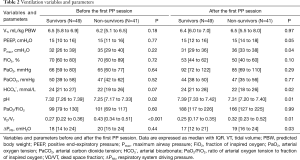
The first PP session resulted in a rise in PaO2/FiO2 that persisted after the patient was repositioned back in the supine position in 90% of the cases, and a decrease in VD/VT and ΔPRS in 66% and 56%, respectively (Figure 2 and Table 2).

Survivors versus non-survivors
PP-induced changes in PaO2/FiO2 and VD/VT were not different between survivors and non-survivors. PP-induced changes in ΔPRS, though, were different between survivors and non-survivors (Figure 2 and Table S3). In the univariate logistic regression, only the absolute PP-induced change in ΔPRS, and the absolute and relative change in arterial pH induced by the first PP session showed an association with 28-day mortality (Table S4). These associations did not sustain in the multivariate analysis when corrected for the propensity score.

Full table
Full table
After repositioning back in the supine position, differences were also noted in arterial pH between survivors and non-survivors (Table S3).
Prognostic value of PP-induced changes in PaO2/FiO2, VD/VT and ΔPRS
Prognostic characteristics of absolute and relative changes in induced by the first PP session are shown in Table 3. Only the absolute PP-induced change in ΔPRS had prognostic capacity, all other PP-induced changes performed poorly. The prognostic capacity for ICU-mortality and 1-year mortality of PP-induced changes were also poor (Table S5,S6).

Full table

Full table
Full table
Discussion
The findings of this post hoc analysis of MARS can best be summarized as follows: (I) PP-induced changes in PaO2/FiO2 and VD/VT are not different between survivors and non-survivors; (II) PP-induced changes in ΔPRS are different between survivors and non-survivors, but the association with outcome is not independent; and (III) prognostic capacity for mortality of PP-induced changes in oxygenation and lung mechanics is insufficient for use in clinical practice.
This is the first study that investigated the prognostic capacities of PP-induced changes in oxygenation, dead space and respiratory system mechanics for outcome prediction in ARDS patients. One strength of this study is that trained researchers collected data used for the calculation of PaO2/FiO2, VD/VT and ΔPRS. In addition, these researchers were also extensively trained in using the diagnostic criteria for ARDS. MARS ran in a tertiary hospital, recruiting a broad selection of ARDS patients, increasing its external validity. Also, different from a recent post hoc analysis of the PROSEVA trial (1), in the current analysis we prevented ‘contamination’ of the early effects of repositioning a patient in the PP by using data from before the first PP session. We also focused on sustained effects of the first PP session, and therefore only used data after repositioning to supine. We consider this is a fairer interpretation of PP-induced changes in gas exchange and lung mechanics.
Though the PP has many benefits, it is still uncertain how ventilation mechanics are exactly altered during repositioning patients from the supine position to the PP, and whether they remain altered after repositioning back to the supine position. Recruitment of lung tissue, changes in intra-abdominal pressures, and chest wall and lung compliances have not been studied well in ARDS patients in the PP, and the exact physiological mechanisms remained uncertain so far. Factors such as weight and pressure relocation, compliance changes, changes in chest wall shape, perfusion and ventilation redistribution have been thought to be contributing factors (25). Even though lung compliance is known to improve in general during a PP session, this does not always happen (25).
While PaO2/FiO2 is used for risk classification in the Berlin Definition, the results of this current study did not find a difference between survivors and non-survivors. This may not be too surprising as the study included patients with more severe ARDS, in which mortality is highest. However, there was also no association between PP-induced changes in PaO2/FiO2 and outcome. This is in line with a previous study investigating the predictive value of PP-induced changes in PaO2/FiO2 (14,26). In that study, mortality rates were similar for ‘responders’ and ‘non-responders’, based on changes in PaO2/FiO2.
In line with a previous investigation (27), VD/VT before the first PP session was lower in survivors compared to non-survivors. Indeed, in that previous study it was shown that higher VD/VT is associated with worse outcome in ARDS patients.
Several recent reports showed an association between ΔPRS and outcome of ARDS patients (11,12,28). The ΔPRS has even been suggested as one of the most important ventilation parameters predicting outcome (4,11,29,30). The results of this current study add to this understanding with the finding that PP-induced changes in ΔPRS were significantly different between survivors and non-survivors. Changes in ΔPRS induced by the first PP session, however, remain to have a disappointing low prognostic capacity. Of note, in all patients PP-induced changes in ΔPRS resulted from a change in Pplat. This can be explained by the fact that the local guidelines for ventilation do not allow changes in PEEP during PP.
In some patients PaO2/FiO2 could have improved beyond the value that triggered the decision to start a PP session. This explains why the PaO2/FiO2 in the last hour before the first PP session was higher than the PaO2/FiO2 that triggered the team to initiate the first PP session, in line with the local guideline for invasive ventilation. Indeed, in between these two-time points changes, and even improvements in PaO2/FiO2 are likely to occur, e.g., due to PEEP titrations or recruitment maneuvers. This finding is in line with what was found in the latest randomized clinical trial of the PP (1).
There are several reasons why we had to reject the hypothesis, apart from the realistic possibility that changes in oxygenation and lung mechanics induced by the first PP session may not be associated with outcome. The sample size of this study was relatively small, and hereby possibly not able to detect the prognostic capacity. It is also possible that changes in PEEP during the first PP session affected the PP–induced changes in the parameters of interest. The findings of the present study may also be seen in light of finding of other studies in which ‘improvements’ in physiologic parameters did not translate in better outcomes, or vice versa. For instance, the seminal RCT comparing low vs. high tidal volumes showed ventilation with a low tidal volume to improve survival, but to worsen oxygenation (31). More recently, a randomized clinical trial of high PEEP and recruitment maneuvers showed the intervention to improve oxygenation and even driving pressure, but to worsen outcome (32). We cannot exclude a similar difference between changes in physiologic parameters and outcomes for positioning in the PP.
This study has several limitations. First, the results must be seen as those from a post hoc analysis. The relative low sample size could limit the generalizability of this analysis. However, the values included in the 95% CI of our primary endpoint (i.e., the AUROC) do not suggest a type II error. It was not possible to determine whether or not patients had received additional recruitment maneuvers during the first PP session, as this was not routinely captured in the database of MARS. Also, esophageal pressure measurements were not performed routinely, and therefore the pulmonary ΔP could not be calculated. Because patients were exclusively under pressure-controlled ventilation, Pmax instead of Pplat was used, as suggested previously (33-35). Furthermore, severity of illness scores like APACHE scores and SAPS have robust prognostic capacities, and thus should be held in consideration when investigating the prognostic capacities of the parameters of interest in this study. However, the multivariate analysis adjusted for a propensity score calculated from age, an age-corrected APACHE IV, and baseline PaO2/FiO2, VD/VT, ΔPRS, VT, and the respiratory rate, also failed to find an association between changes in the parameters and outcomes. Finally, the study focused on the first PP session and not successive sessions.
Conclusions
In conclusion, in this cohort of ARDS patients the first PP session induced changes in PaO2/FiO2, VD/VT, and ΔPRS. Only changes in ΔPRS were different between survivors and non-survivors. Neither the change in PaO2/FiO2 and VD/VT, nor in ΔPRS induced by the first PP session had sufficient prognostic capacities for outcome.
Acknowledgments
Funding: This work was supported by the Center for Translational Molecular Medicine (www.ctmm.nl) (grant 041201).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Institutional Review Board approved the study protocol of the parent study and the use of an opt-out consent procedure (protocol no. 10-056C).
References
- Guérin C, Reignier J, Richard JC, et al. Prone Positioning in the Acute Respiratory Distress Syndrome. N Engl J Med 2013;369:980-1. [PubMed]
- Beitler JR, Shaefi S, Montesi SB, et al. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med 2014;40:332-41. [Crossref] [PubMed]
- Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone Position for Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann Am Thorac Soc 2017;14:S280-8. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Guérin C, Beuret P, Constantin JM, et al. A prospective international observational prevalence study on prone positioning of ARDS patients: the APRONET (ARDS Prone Position Network) study. Intensive Care Med 2018;44:22-37. [Crossref] [PubMed]
- Gattinoni L, Tognoni G, Pesenti A, et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 2001;345:568-73. [Crossref] [PubMed]
- Kallet RH, Zhuo H, Liu KD, et al. The Association Between Physiologic Dead-Space Fraction and Mortality in Subjects With ARDS Enrolled in a Prospective Multi-Center Clinical Trial. Respir Care 2014;59:1611-8. [Crossref] [PubMed]
- The ARDS Definition Task Force. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Raurich JM, Vilar M, Colomar A, et al. Prognostic value of the pulmonary dead-space fraction during the early and intermediate phases of acute respiratory distress syndrome. Respir Care 2010;55:282-7. [PubMed]
- Lucangelo U, Bernabè F, Vatua S, et al. Prognostic value of different dead space indices in mechanically ventilated patients with acute lung injury and ARDS. Chest 2008;133:62-71. [Crossref] [PubMed]
- Amato MBP, Meade MO, Slutsky AS, et al. Driving Pressure and Survival in the Acute Respiratory Distress Syndrome. N Engl J Med 2015;372:747-55. [Crossref] [PubMed]
- Laffey JG, Bellani G, Pham T, et al. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med 2016;42:1865-76. [Crossref] [PubMed]
- Gattinoni L, Pesenti A, Carlesso E. Body position changes redistribute lung computed-tomographic density in patients with acute respiratory failure: impact and clinical fallout through the following 20 years. Intensive Care Med 2013;39:1909-15. [Crossref] [PubMed]
- Albert RK, Keniston A, Baboi L, et al. Prone position-induced improvement in gas exchange does not predict improved survival in the acute respiratory distress syndrome. Am J Respir Crit Care Med 2014;189:494-6. [Crossref] [PubMed]
- Klein Klouwenberg PMC, Ong DSY, Bos LDJ, et al. Interobserver agreement of Centers for Disease Control and Prevention criteria for classifying infections in critically ill patients. Crit Care Med 2013;41:2373-8. [Crossref] [PubMed]
- Bos LD, Cremer OL, Ong DS, et al. External validation confirms the legitimacy of a new clinical classification of ARDS for predicting outcome. Intensive Care Med 2015;41:2004-5. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453-7. [Crossref] [PubMed]
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818-24. [Crossref] [PubMed]
- Schultz MJ, de Pont AC. Prone or PEEP, PEEP and prone. Intensive Care Med 2011;37:366-7. [Crossref] [PubMed]
- Determann RM, Royakkers A, Wolthuis EK, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Critical Care 2010;14:R1. [Crossref] [PubMed]
- Haukoos JS, Lewis RJ. The Propensity Score. JAMA 2015;314:1637-8. [Crossref] [PubMed]
- Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373-9. [Crossref] [PubMed]
- Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32-5. [Crossref] [PubMed]
- Hajian-Tilaki K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Caspian J Intern Med 2013;4:627-35. [PubMed]
- Pelosi P, Tubiolo D, Mascheroni D, et al. Effects of the Prone Position on Respiratory Mechanics and Gas Exchange during Acute Lung Injury. Am J Respir Crit Care Med 1998;157:387-93. [Crossref] [PubMed]
- Gattinoni L, Vagginelli F, Carlesso E, et al. Decrease in PaCO2 with prone position is predictive of improved outcome in acute respiratory distress syndrome. Crit Care Med 2003;31:2727-33. [Crossref] [PubMed]
- Nuckton TJ, Alonso JA, Kallet RH, et al. Pulmonary dead-spae fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 2002;346:1281-6. [Crossref] [PubMed]
- Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998;338:347-54. [Crossref] [PubMed]
- Neto AS, Hemmes SNT, Barbas CSV, et al. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. Lancet Respir Med 2016;4:272-80. [Crossref] [PubMed]
- Bugedo G, Retamal J, Bruhn A. Driving pressure: a marker of severity, a safety limit, or a goal for mechanical ventilation? Crit Care 2017;21:199. [Crossref] [PubMed]
- The ARDS Network. Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. N Engl J Med 2000;14:343:813.
- Cavalcanti AB, Suzumura ÉA, Laranjeira LN, et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP vs Low PEEP on Mortality in Patients Witch Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2017;318:1335-45. [Crossref] [PubMed]
- Bos LD, Schouten LR, Cremer OL, et al. External validation of the APPS, a new and simple outcome prediction score in patients with the acute respiratory distress syndrome. Ann Intensive Care 2016;6:89. [Crossref] [PubMed]
- Chatburn RL, Volsko TA. Documentation issues for mechanical ventilation in pressure-control modes. Respir Care 2010;55:1705-16. [PubMed]
- Becher T, van der Staay M, Schädler D, et al. Calculation of mechanical power for pressure-controlled ventilation. Intensive Care Med 2019;45:1321-3. [Crossref] [PubMed]


