
Sublobar resection: functional evaluation and pathophysiological considerations
Introduction
Pulmonary function tests (PFTs) are widely used by thoracic surgeons to predict post-operative outcomes and complications for patients undergoing a pulmonary resection. Predicted postoperative forced expiratory volume in one second (ppoFEV1) is the most widely used parameter to assess the preoperative risk, but a single value of FEV1 without a correlation to other functional parameters may be insufficient and insidious.
The present critical review will analyze how PFTs are currently used in clinical practice and what kind of information they may provide to clinicians and surgeons to personalize the management of patients undergoing lung surgery based on their functional characteristics.
Thoracic surgery operability criteria: state of the art
Every kind of surgical intervention can affect the lung: supine position and general anaesthesia produce a headward shift of the diaphragm and reduce the vital capacity, the tidal volume and the respiratory rate. Cough reflex is abolished and atelectasis may occur in depending regions when mechanical ventilation is not well titrated. For patients undergoing thoracic surgery, lobar or lung resection can magnify all the complications from general anaesthesia and general surgery.
British Thoracic Society (BTS) guidelines for lung cancer surgery recommend to perform lung function tests with lung diffusion capacity for carbon monoxide (DLCO) and arterial blood gas analysis in all patients that undergo thoracic surgery (1). Patients are defined as operable with an average operative risk when FEV1 is >1.5 L (>2 L for pneumonectomy) or ppoFEV1 is ≥40%, DLCO is ≥40% predicted and oxygen saturation >90%. According to the BTS guidelines, when one or more of these criteria are not satisfied, additional tests should be performed, e.g., a 6-minute walk test, a cardiopulmonary exercise test or a quantitative perfusion scan. A perfusion scan provides an accurate measurement of the ppoFEV1, although the latter can be estimated by some mathematic equations (1). The exercise test is very useful when spirometric criteria are not reached: BTS guidelines identify the peak rate of oxygen consumption (VO2) as the most important parameter for the operability evaluation. The threshold for VO2 defined by guidelines for an “average operative risk” is VO2 >15 mL/kg/min, otherwise, patients are classified in the “high risk” class.
The choice of the type of intervention, lobectomy vs. segmentectomy or other minimal resections, belongs to thoracic surgeons and it is not within the scope of the present review, which will focus only on the functional evaluation of pre-operatory and post-operative lung pathophysiology.
Functional consideration in real life: a literature analysis
Guidelines worldwide recommend performing a complete bundle of PFTs; nevertheless, in the clinical and surgical practice FEV1 is the parameter most frequently used, and often the only parameter considered for the indication to operate the patient. Ppo-FEV1 has proved to be an independent risk factor for post-operatory morbidity and mortality, but according to guidelines, all functional variables should be considered to assess the phenotype of every single patient and to guide clinicians and surgeons towards the best management of each cases, “personalizing” as much as possible the surgery indication (1).
The literature comparing lung function after lobectomy with minor resections such as segmentectomy is scarce and investigations are difficult to compare due to the inhomogeneity of the patients enrolled and the variability of the lung function parameters reported. The major limitations of the studies we report are represented by the retrospective design and the lack of patients’ clinical and pathophysiological characterization
Almquist and colleagues enrolled 149 patients and studied how preoperative FEV1 and DLCO impacted on short- and long-term outcomes after lung surgery for early stage non-small cell lung cancer (NSCLC stage I and II) (2). Most of the patients underwent lobectomy, but patients with wedge resections and pneumonectomy were also included. Authors performed a multivariate analysis to study predictors of mortality and of length of hospitalization: neither FEV1 nor DLCO resulted to be good predicting factors for mortality, although they both were independent risk factors for the length of hospitalization (in latter case, more than 10 days).
In the study by Almquist et al., the FEV1 to forced vital capacity (FVC) ratio was not reported, therefore it was not possible to verify if this cohort of patients had an obstructive, a restrictive or a normal lung function pattern.
In a prospective study of patients with early stage NSCLC and a good operatory risk, Takizawa and colleagues enrolled 184 patients: 133 underwent lobectomy and 51 segmentectomy (3). The aim of the study was to investigate the post-operative functional advantage of performing segmentectomy and wedge resection compared with lobectomy. PFTs were performed at baseline (pre-operatory) and then 2 weeks and 12 months after the resection. Pre-operative FVC and FEV1 were not significantly different between the two groups and the mean FEV1/FVC ratio that could be inferred from the available data was generally within normal range, allowing us to speculate that the basal and pre-operative functional pattern of the study sample was normal.
Nevertheless, no significant difference in absolute FVC value was observed between the two groups either 2 weeks or 12 months after the surgery (mean ± standard deviation; segmentectomy vs. lobectomy: 2.05±0.66 vs. 1.91±0.51 liters, P=0.3 and 2.67±0.73 vs. 2.57±0.59 liters, P=0.5 respectively after 2 weeks and 12 months), but while the total loss in FVC after 12 months in the lobectomy group was 280 mL, patients that underwent segmentectomy lost 140 mL (3).
Considering the post-operative FEV1 value, the between group difference both at 2 weeks and 12 months was of 40 mL; however, 2 weeks after surgery, the total loss of FEV1 in the lobectomy group was of 750 mL, while in the segmentectomy group was of 590 mL; the total re-gain in FEV1 12 months after the intervention was comparable, around 450 mL, without reaching the pre-surgery value (3). Unfortunately, the authors did not compare the pre and post-surgery parameters after 2 weeks and 12 months, thus the presence of a significant difference between the two approaches cannot be inferred. However, in this study the segmentectomy procedure seems to give a functional advantage both for FVC and FEV1, although the comparison between the two groups did not reach a statistical difference. In a retrospective study conducted by Deng and co-workers, lobectomy and segmentectomy procedures were compared in terms of 5 years overall survival, disease-free survival and post-operative lung function in early stage NSCLC patients (4). The advantage of different surgical approaches in terms of post-surgery survival still represents an open issue and will not be the subject of the present review.
The lung function parameters reported were the mean FEV1, FVC and DLCO, performed at baseline (pre-operatory) and within 24 months after surgery. No FEV1/FVC ratio was reported. The most frequent comorbidity was COPD, 43.6% and 60.8% in patients treated with lobectomy and segmentectomy, respectively. At the end of the follow-up period, the surgical approach appeared not to have influenced the post-operative lung function, with no difference in FVC, FEV1 and DLCO in patients that underwent lobectomy or segmentectomy. Conversely, Kashiwabara and colleagues compared pre-operative and 6 months post-operative FEV1 in patients with early stage NSCLC that underwent lobectomy or segmentectomy (5). In their study, patients were stratified in two groups: patients with a ppoFEV1 <70% and with a ppoFEV1 ≥70% of predicted value.
In patients with ppoFEV1 <70%, there was no significant difference in the total loss of FEV1 between the segmentectomy and the lobectomy group; vice versa, in patients with a preserved ppoFEV1, the ΔFEV1 after the surgery was smaller in the segmentectomy group, suggesting a functional advantage over lobectomy (5).
Functional considerations
None of the cited studies reported the FEV1/FVC, the parameter that is essential to phenotype the functional pattern of the patients enrolled, if obstructive, restrictive or normal. A thorough pre-operative patients’ functional evaluation represents a step of paramount importance in view of the selection of the best surgical approach and strategy, to drive and the best pre- and post-surgical management of patients with lung cancer.
In Takizawa’s study, the loss of FVC and FEV1 12 months after a lobectomy is almost twice as much as compared with segmentectomy (mean loss in FVC at 12 months: −140 and −280 mL; mean loss in FEV1: −140 and −300 mL, for segmentectomy and lobectomy, respectively) (3). The FVC depends on the lung-chest wall compliance and airways size: when a larger anatomic part is removed, more parenchyma and elastic fibres are lost and a bigger scar is performed. Thus, less amount of FVC lost in patients undergoing a segmentectomy might be explained by the minor resection and a reduced loss in lung compliance. This is sustained by the measurements of lung mechanics performed by Berend and colleagues in patients treated with lobectomy (6).
Patients undergoing segmentectomy appeared to have also a functional advantage in terms of post-operative FEV1. The latter may be justified by the size and number of resected airways, so that the total size of the airways could have an impact on the resulting FEV1. However, the reviewed studies show contrasting results in terms of FEV1 post-surgical loss and recovery due to important differences in the baseline patients characteristics. All considered, the isolated FEV1 value seems not to be a good predictor of the lung function trajectory in patients undergoing lobectomy and segmentectomy.
When the loss of FEV1 and FVC is analysed 2 weeks after the surgery and then after 12 months, there is a re-gain of about 650 mL in FVC and 450 mL in FEV1 for both the surgical approaches. The stress applied by surgery to the chest wall (rib-cage and thoracic muscles) and to the pleura and lung appears to impact the thoraco-pulmonary system compliance, such as the elastic properties are “paralyzed” and then resumed after 12 months. Considering an unaltered total lung capacity (TLC) after the surgical intervention, with no scarring tissue to create an extra-pulmonary restrictive pattern per se, the lung parenchyma re-expands to fill the room left in the chest cavity. However, we may expect that the mechanics of the respiratory system could adapt differently, for any given different pre-operative parenchymal condition (presence of emphysema, flow limitation, fibrosis etc.) and depending on the regional distribution of the parenchymal disease.
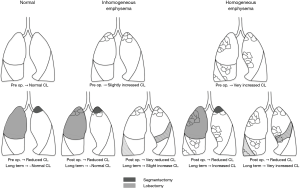
In the study by Kashiwabara and colleagues (5) the majority of the study sample is represented by smokers, while the 16% of the patients in the lobectomy arm and 20% of the segmentectomy arm had emphysema. The authors observe that only patients without pre-procedural airflow obstruction (a ppoFEV1 <70% of the predicted value) exposed to lobectomy suffer from a reduction in FEV1 and FVC after surgery. These findings deserve a short pathophysiological parenthesis to explain the possible effects of lung surgery on lung mechanics, especially in patients with airflow obstruction. Patients with COPD are characterized by maldistribution of ventilation with impaired gas exchange and peripheral lung injury, these abnormalities due to the presence of closing volume, the lung volume at which some regions of the lung stop contributing to lung deflation and trap air beyond functionally closed airways (7). Roughly half of patients with COPD independently of disease severity, experience a cyclic opening and closure of the small airways during tidal breathing because the closing volume exceeds the functional residual capacity (8), a phenomenon which can be partially reversed by the administration of bronchodilators (9). When patients with COPD undergo a lung excision—e.g., a lobectomy—all static lung volumes tend to decrease (TLC, FRC and residual volume), together with a decrease in lung compliance (6); due to the reduction of FRC, some of these patients experience also an increase in closing volume (6), a condition that worsens the stresses implicated in lung ventilation and promotes the damage to the remaining airways (10,11). In the study by Kashiwabara and coworkers, the majority of patients with emphysema had an upper lobe distribution of the disease, with the lobectomy mostly directed to upper lobes. We can speculate that the removal of an emphysematous parenchyma may have caused a partial improvement of the regional lung volume distribution and ventilation inhomogeneity, thus explaining the lack of difference in lung function loss compared with patients with a preserved FEV1. The type and extent of lung resection should therefore be carefully studied and personalized in view of the pathophysiological characterisation of each patient, that should be as complete as possible, including static, dynamic lung volumes and ventilation inhomogeneity. In Figure 1, we report a proposal for a schematic approach to lobectomy and segmentectomy depending on the presence and distribution of emphysema.
DLCO represents a very important parameter to assess patients’ operability: as discussed before, according to BTS guidelines patients are classified in the “average risk” class when DLCO is ≥40% predicted value (1). As it happens for static volumes, patients may have different causes for a reduction in pre-operative DLCO [ventilation inhomogeneity vs. vascular/epithelial or alveolar damage (12)], which may have a different impact on their exercise capacity and gas exchange before and after surgery (6). Therefore, the type of surgical approach should also rely on a careful evaluation of the DLCO pattern. Not surprisingly, the pre-operative DLCO has been demonstrated to predict the risk of complications, short and long-term outcomes and the length of hospitalization in patients that underwent thoracic surgery (2).
In conclusion, the isolated value of FEV1 is not a reliable parameter to guide the selection of patients for lung surgery. Spirometric data should be always associated to other lung function parameters such as static volumes, airway resistances and indexes of ventilation distribution, in order to improve the pathophysiological assessment and the careful evaluation of the functional pattern in patients undergoing procedures. Specifically, designed and prospective studies are needed to establish the relationship and long-term consequences of different surgical approaches in terms of lung mechanics and functional loss.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mario Nosotti, Ilaria Righi and Lorenzo Rosso) for the series “Early Stage Lung Cancer: Sublobar Resections are a Choice?” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2019.12.35). The series “Early Stage Lung Cancer: Sublobar Resections are a Choice?” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- British Thoracic Society, Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party. BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax 2001;56:89-108. [Crossref] [PubMed]
- Almquist D, Khanal N, Smith L, et al. Preoperative Pulmonary Function Tests (PFTs) and Outcomes from Resected Early Stage Non-small Cell Lung Cancer (NSCLC). Anticancer Res 2018;38:2903-7. [PubMed]
- Takizawa T, Haga M, Yagi N, et al. Pulmonary function after segmentectomy for small peripheral carcinoma of the lung. J Thorac Cardiovasc Surg 1999;118:536-41. [Crossref] [PubMed]
- Deng B, Cassivi SD, de Andrade M, et al. Clinical outcomes and changes in lung function after segmentectomy versus lobectomy for lung cancer cases. J Thorac Cardiovasc Surg 2014;148:1186-92.e3. [Crossref] [PubMed]
- Kashiwabara K, Sasaki J, Mori T, et al. Relationship between functional preservation after segmentectomy and volume-reduction effects after lobectomy in stage I non-small cell lung cancer patients with emphysema. J Thorac Oncol 2009;4:1111-6. [Crossref] [PubMed]
- Berend N, Woolcock AJ, Marlin GE. Effects of lobectomy on lung function. Thorax 1980;35:145-50. [Crossref] [PubMed]
- Milic-Emili J, Torchio R, D'Angelo E. Closing volume: a reappraisal (1967-2007). Eur J Appl Physiol 2007;99:567-83. [Crossref] [PubMed]
- Pecchiari M, Radovanovic D, Santus P, et al. Airway occlusion assessed by single breath N2 test and lung P-V curve in healthy subjects and COPD patients. Respir Physiol Neurobiol 2016;234:60-8. [Crossref] [PubMed]
- Pecchiari M, Santus P, Radovanovic D, et al. Acute effects of long-acting bronchodilators on small airways detected in COPD patients by single-breath N2 test and lung P-V curve. J Appl Physiol 1985;2017:1266-75. [PubMed]
- Santus P, Pecchiari M, Tursi F, et al. The Airways' Mechanical Stress in Lung Disease: Implications for COPD Pathophysiology and Treatment Evaluation. Can Respir J 2019;2019:3546056. [Crossref] [PubMed]
- Kirby M, Tanabe N, Tan WC, et al. Total Airway Count on Computed Tomography and the Risk of Chronic Obstructive Pulmonary Disease Progression. Findings from a Population-based Study. Am J Respir Crit Care Med 2018;197:56-65. [Crossref] [PubMed]
- Santus P, Radovanovic D, Balzano G, et al. Improvements in Lung Diffusion Capacity following Pulmonary Rehabilitation in COPD with and without Ventilation Inhomogeneity. Respiration 2016;92:295-307. [Crossref] [PubMed]


