Effect of a neutrophil elastase inhibitor on ventilator-induced lung injury in rats
Introduction
Mechanical ventilation (MV) can initiate as well as exacerbate lung injury, which is referred to as ventilator-induced lung injury (VILI). As well as causing lung injury, it can also decrease the functioning of other organs and contribute to mortality (1). VILI can lead to remote organ dysfunction and multiple organ failure (2).
Multifactorial etiologies of VILI, from either direct or indirect injury to the lung, have been postulated (3). Diffuse alveolar damage and initial vascular leak with a neutrophil-predominant inflammatory response are the key features of VILI (4). High-tidal-volume (VT) MV has been proved to induce the activation of proinflammatory cytokines, and thus lead to VILI (5). MV with high tidal volumes and pressure can lead to increased alveolar-capillary permeability accompanied by the release of pro-inflammatory mediators by the lung cells in response to mechanical stretch. These stimuli trigger detachment of endothelial cells from the basement membrane and synthesis of extracellular matrix components (6). Injurious MV also promotes alveolar coagulopathy and fibrin deposition within the airways (7). Additionally, the generation of reactive oxygen species (ROS) during VILI causes direct cellular injury and triggers ROS-sensitive, aberrant activation of cellular mechanisms leading to severe inflammation, resulting in rapid transcription of pro-inflammatory cytokines and chemokines (8). Neutrophils accumulate in the microvasculature of injured lungs, releasing various cytokines, chemokines and proteases. Among these proteins, the activity of neutrophil elastase is increased in patients with adult respiratory distress syndrome (9).
Neutrophil elastase, located downstream in the humoral mediator network, contributes to the development of vascular endothelial injury in concert with other mediators, which leads to increased permeability, vasodilation, and activation of the coagulation cascade (10). Furthermore, this pulmonary pro-inflammatory response is not confined to the lungs and extends into the systemic circulation, contributing to the development of systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS) (11). Modulating this imbalance between pro- and anti-inflammatory mediators in the lung could be used as a therapeutic approach.
Sivelestat {ONO-5046; sodium N-[2-[4-(2,2-dimethylpropionyloxy) phenylsulfonylaminobenzoyl]aminoacetate tetrahydrate]} is a specific inhibitor of neutrophil elastase, discovered and characterized by Ono Pharmaceutical Co. Ltd. in Japan (12). Investigators have reported that the neutrophil elastase inhibitor sivelestat plays protective roles in the lung with ischemia-reperfusion injury (13), and lipopolysaccharide-induced injury (14).
Recently, it has been reported that post-operative sivelestat administration after transthoracic esophagectomy improved the pathophysiological condition of SIRS and the post-operative clinical course even in patients without complications, as well as the deterioration of the PaO2 (arterial oxygen tension)/FiO2 (inspired oxygen fractional concentration) ratio in the post-operative period following esophagectomy (15), and surgery for congenital heart disease with pulmonary hypertension (16). However, its prophylactic effect on VILI is unknown. Sakashita showed that neutrophil elastase inhibitor, given 30 min before ventilation, suppressed subsequent neutrophilic inflammation, attenuating the histopathological degree of lung damage, lowering neutrophil accumulation and lung water content, induced by VILI with high tidal volume ventilation in the C57/BL6 mice model (17).
We hypothesized that pretreatment with neutrophil elastase inhibitor (sivelestat) would decrease ventilator-induced microvascular permeability, recruitment of neutrophils and oxidative injury. To test this hypothesis, we ventilated with high tidal volume and examined the effects of sivelestat in preventing acute lung injury (ALI) induced by VILI in a rat model and examined its effect on lung injury and compared ALI parameters with those resulting from prophylactic sivelestat and routine sivelestat. This study explored the role of the protective effect of sivelestat in lung injury induced by VILI in a rat model and to provide theoretical basis for clinical treatment.
Methods
Animal preparation
Eight-week-old Sparague-Dawley rat weighing 200-300 g were used. All animals received humane care, in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health. Our protocol and experimental methods were approved by the Animal Care and Use Committee of the Laboratory Animal Service of the University of Pusan University School of Medicine. All surgery was performed under ketamine anesthesia and all efforts were made to minimize suffering.
Experimental groups
Anesthetized rats received MV with a high tidal volume (20 mL/kg) for 3 h. The neutrophil elastase inhibitor (sivelestat, 100 mg/kg) was administered intraperitoneally (i.p.) 30 min before and at the initiation of ventilation. The rats were categorized as (I) sham group: injected with saline (10 mg/kg body, i.p.) at the time of ventilation start; (II) VILI group; (III) sivelestat group: sivelestat (100 mg/kg body, i.p.) at the initiation of ventilation; (IV) early sivelestat group: sivelestat (100 mg/kg body, i.p.) 30 min before ventilation and at the initiation of ventilation.
Rats subjected to sham, VILI, sivelestat and early sivelestat group were injected with an identical volume of placebo-control solution or sivelestat. In each group, underwent measurements for lung water content, bronchoalveolar lavage fluid (BALF) neutrophil and BALF protein, MDA, hematoxylin and eosin (H&E) stain.
Murine model of VILI
We used our established mouse model of VILI as previously described (18). Rats were anesthetized i.p. with ketamine (100 mg/kg; Eurovet Animal Health BV, Bladel, the Netherland). After inserting a 22-gauge ventilation cannula into the trachea, the rats were placed in the supine position on a warming device and then connected to a ventilator. The VILI model was defined as a high VT of 20 mL/kg, a respiratory rate of 60 breaths per min and MV maintained for 3 h at an inspiratory oxygen fraction of 0.21. The ventilator was a model 683 from Harvard Apparatus Co. (Harvard Apparatus, Holliston, MA, USA). Sham group, the rats were injected with saline (10 mg/kg body, i.p.) at the time of ventilation start. Sivelestat pretreated VILI group were injected with sivelestat (100 mg/kg body, i.p.) 30 min before ventilation and sivelestat treated VILI group were sivelestat (100 mg/kg body, i.p.) at the time of ventilation start.
Lung water content
Lung water content related to lung injury was measured using the lung wet-to-dry weight ratio as described (19). The left lower lungs were weighed immediately after removal (wet weight) and again after drying in an oven at 60 °C for 72 h (dry weight). The lung wet-to-dry weight ratio was calculated as the ratio of wet weight to dry weight. Lung wet-to-dry weight ratio was used as an index of pulmonary edema formation.
Bronchoalveolar lavage fluid (BALF) protein levels
After MV for 3 h, the right lung was used for bronchoalveolar lavage via slow intratracheal injection of two sequential 1-mL aliquots of sterile normal NaCl. Bronchoalveolar lavage was performed twice using 1 mL of saline. Total cell counts were determined using standard hematological procedures in 40-µL aliquots of each sample. The remaining fluid was centrifuged at 1,500 rpm for 10 min (at 4 °C) and the supernatant was stored at –80 °C until analysis. The protein quantification method using bicinchoninic acid was developed by Smith et al. (20). The Lowry assay (21) and this assay are of similar sensitivity, but this assay is stable under alkali conditions and can be carried out as a one-step process compared to the two steps needed in the Lowry assay. Total protein concentration in the BALF was determined with the WelProtTM Protein Assay Kit (Welgene, Daegu, Korea) according to the manufacturer’s instructions.
Bronchoalveolar lavage fluid (BALF) neutrophil counts
BALF was obtained from the right lung and cell counts were determined using a hemocytometer (Beckman Coulter, Fullerton, CA, USA). Differential counts were performed on cytospin preparations stained with a modified Giemsa stain, Diff-Quick (Dade Behring AG, Düdingen, Switzerland).
Malondialdehyde (MDA) assay
Oxidative injury from VILI was determined by measuring the tissue concentration of MDA, a marker of lipid peroxidation in lung tissue. MDA, which is an end product of lipid peroxidation, was estimated using the Oxiselect TBARS assay kit containing thiobarbituric acid-reactive substances (Cell Biolabs, San Diego, CA, USA) according to the manufacturer’s instructions. The MDA concentration was calculated using the absorbance coefficient of the MDA-TBA complex (absorbance coefficient ε=1.565 cm–1.M–1) and is expressed as nanomoles per milligram of protein (nmol/mg protein).
Histopathological grading of VILI
For histological examination, the right upper lobe of the lung was fixed in 10% neutral-buffered formalin, embedded in paraffin, and sectioned at 4 µm thickness. After deparaffinization and dehydration, the sections were stained with H&E for microscopic examination.
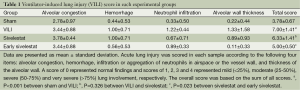
The lungs were reviewed from five non-overlapping fields by a single pathologist (Korea CFC Pathology Laboratory, Jin Kyu Park) blinded to the therapeutic category of the rat. A modified VILI histological scoring system was applied as described (22). Lung injury was scored on a scale of 0-4 using the average score of the following items: (I) alveolar capillary congestion; (II) hemorrhage; (III) infiltration of neutrophils into the airspace or the vessel wall and thickness of the alveolar wall; and (IV) alveolar wall thickness/hyaline membrane formation.
A score of 0 represented normal findings and scores of 1, 2, 3 and 4 represented mild (<25% lung involvement), moderate (25-50% lung involvement), severe (50-75% lung involvement) and very severe (>75% lung involvement) lung involvement, respectively. The overall score was based on the sum of all scores.
Statistical analysis
Data are expressed as means ± standard deviation (SD). When data were not normally distributed according to a Kolmogorov-Smirnov test, we used Wilcoxon signed-rank test. Differences among groups were analyzed using a one-way analysis of variance or the Kruskal-Wallis test. When differences were statistically significant, individual results were compared using the Bonferroni test. The Kruskal-Wallis test was used for the analysis of VILI score. A P value of <0.05 was considered to indicate significance. Statistical analyses were performed using SPSS for Windows (version 20.0; SPSS Inc.; Chicago, IL, USA).
Results
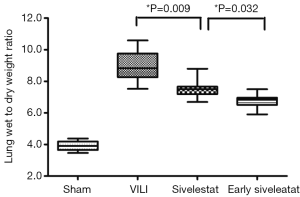
Sivelestat decreases lung edema induced by VILI
The ratio of wet-to-dry weight, a parameter of lung edema, was significantly increased in the VILI group (Figure 1; 9.05±1.02, P<0.001). This increase was significantly attenuated in the Early sivelestat group (6.76±2.01) compared with the Sivelestat group (7.39±0.32, P=0.036). These data indicate that early administration of sivelestat inhibited lung edema induced by high VT ventilation.
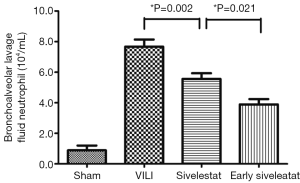
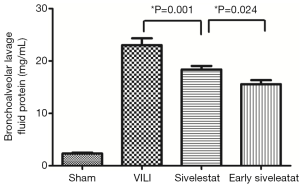
Effect of sivelestat on BALF parameters (alveolar-capillary leak due to VILI)
In the VILI group, the BALF neutrophil counts (Figure 2), and protein concentration (Figure 3) were significantly increased. BALF neutrophil [(0.89±0.93)×104vs. (7.67±1.41)×104 cells/mL, respectively; P<0.001] and protein (2.34±0.47 vs. 23.01±3.96 mg/mL, respectively; P<0.001) levels were significantly higher in the VILI group compared to the sham group. The early sivelestat group exhibited reduced increases in protein concentration (18.38±2.00 vs. 15.57±2.32 mg/mL, respectively; P=0.024), neutrophil count [(5.56±1.13)×104vs. (3.89±1.05)×104 cells/mL, respectively; P=0.021) in BALF compared to the sivelestat group. These findings suggest that early sivelestat administration inhibits the subsequent development of VILI, including neutrophil accumulation.
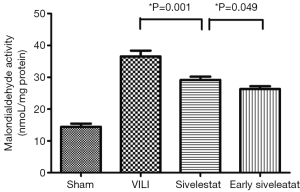
Effect of sivelestat on lung lipid peroxidation
The concentration of MDA, an indicator of the degree of lipid peroxidation, was increased to a significantly lesser extent in the early sivelestat group (Figure 4). Tissue MDA was significantly higher in the VILI group than the Sham group MDA (14.43±1.01 vs. 36.56±5.45 nmol/mg protein, respectively; P=0.001). The early sivelestat group exhibited a reduced increase in MDA concentration (29.16±3.01 vs. 26.31±2.58 nmol/mg protein, respectively; P=0.049) compared with the Sivelestat group. These findings suggest that sivelestat inhibits the subsequent development of VILI, including lipid peroxidation.
Histological assessment of lung injury
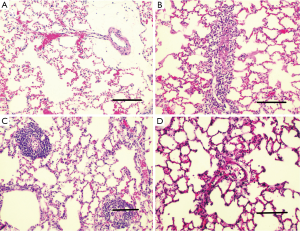
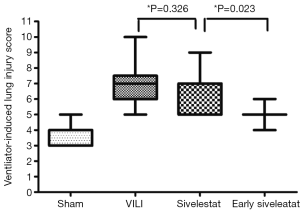
H&E-stained lung sections of each experimental group are shown in Figure 5: sham group (A), VILI group (B), sivelestat group (C) and early sivelestat group (D). The images are representative histological lung tissue sections (magnification, ×100, H&E stain). Perivascular and interstitial edema, inflammatory cell infiltration, intra-alveolar hemorrhage, and hyaline membrane formation or wall thickness of lung architecture were detected in the lungs of the VILI animals. The lungs of the early sivelestat-treated animals showed minor signs of pulmonary edema and cell influx. Figure 6 shows the histopathological grades of VILI using the scoring system. The VILI scores were significantly increased in the VILI group compared with the sham group (Table 1, 3.78±0.67 and 7.00±1.41, respectively, P<0.001). The scores in the Early sivelestat group were significantly lower than those in the Sivelestat group (6.33±1.41 and 5.00±0.50, respectively, P=0.023). Early administration of sivelestat successfully reduced the histopathological severity of lung injury.
Full table
Discussion
We have shown that pretreatment with sivelestat attenuates the physiological, biochemical, and histological deterioration in the high-VT-induced VILI rat model. Sivelestat attenuated the pulmonary edema, as shown by the amelioration of the increased wet/dry-weight ratio and BALF protein levels. The protective effect of sivelestat may be due, in part, to protection of the pulmonary endothelium from the elastase released by neutrophils. This indicates that the drug also attenuated the accumulation of neutrophils in the lung.
MV causes the activation of macrophages, which promotes the migration of neutrophils from blood vessels into the alveolar space and discharge of neutrophil elastase and oxidants. Alveolar-capillary membranes are subsequently damaged and activated neutrophils induce an increase in lung vascular permeability that causes protein-rich lung edema (23). Neutrophil elastase is a protease that can degrade key structural elements of connective tissue, such as elastin, collagen, and proteoglycan (24). Neutrophil elastase has been implicated in the increase in permeability in both vascular endothelial (25) and alveolar epithelial (26) cells that are involved in lung edema. Additionally, it has been reported that sivelestat indirectly reduces neutrophil sequestration by suppressing the release of neutrophil chemotactic factors (IL-8) (13), inflammatory cytokines (IL-6, TNF-α) (12), and other adhesion-promoting molecules (P-selectin, intercellular adhesion molecule-1) (27). These findings have strengthened the notion that MV can lead to the infiltration of neutrophils, and that neutrophils become targets of neutrophil elastase during the pathogenesis of VILI. Previous studies reported that sivelestat reduced lung injury associated with SIRS in humans and decreased endotoxin-induced lung injury in animal models (28). Neutrophil elastase is known to play a central role in the pathophysiology of ALI, not only by causing direct tissue injury but also by amplifying the inflammatory response. Sivelestat improved the arterial oxygen tension to the inspired oxygen fraction (PaO2/FiO2) and decreased the length of stay in the intensive care unit and days on a ventilator for patients with ALI and acute respiratory distress syndrome (ARDS) (29). Moreover, treatment of ARDS with sivelestat reduced serum cytokine levels (30). Several animal studies have shown neutrophil elastase inhibition to be effective (13,31-34). However, sivelestat has not been shown to suppress the release of cytokines associated with VILI, and no reliable, conclusive clinical study has yet been conducted (35). In the present study, we pretreated animals with neutrophil elastase inhibitor and ventilated them with injurious VT. By contrast with lung injuries in previous studies, induced by intraperitoneal endotoxin, ischemia-reperfusion, and thrombin (13,31-34), we induced lung injury by high VT ventilation that continued to injure the lungs during ventilation compared to previous studies.
Lipid peroxidation is a well-defined mechanism of cellular damage in animals and plants. Lipid peroxides are unstable indicators of oxidative stress in cells that decompose to form more complex and reactive compounds such as MDA and 4-hydroxynonenal, natural bi-products of lipid peroxidation. Oxidative modification of lipids can be induced in vitro by a wide array of pro-oxidant agents and occurs in vivo during aging and in certain disease conditions. Measuring the end products of lipid peroxidation is one of the most widely accepted assays of oxidative damage. These aldehydic secondary products of lipid peroxidation are generally accepted markers of oxidative stress. Recent reports have shown that oxygen radicals released from neutrophils might contribute to the development of acute edematous lung injury in isolated rabbit lungs (36) and antioxidants attenuated ALI in isolated perfused rat lungs (37). Our findings showed that the concentration of MDA, an indicator of the degree of lipid peroxidation, was increased to a significantly lesser extent in the Early sivelestat group. Therefore, the antioxidant role of sivelestat is anticipated to prevent the development of ALI in our experimental system.
A Japanese phase III trial demonstrated that sivelestat was effective for patients with ALI associated with SIRS, showing increased pulmonary oxygenation ability, a decrease in the duration of MV, and a shortened stay in the intensive care unit (ICU) (13). It has been reported recently that post-operative sivelestat administration after transthoracic esophagectomy improved the pathophysiological condition of SIRS and the postoperative clinical course even in patients without complications, as well as the deterioration of the PaO2/FiO2 ratio in the postoperative period following esophagectomy (15), surgery for congenital heart disease with pulmonary hypertension (16). However, its prophylactic effect on VILI is still unknown. In accordance of Sakashita et al. study (17), our study showed that prophylactic sivelestat attenuate VILI. Additionally, our study showed that prophylactic sivelestat administration attenuate VILI more effectively, compared to conventional sivelestat administration. This interesting finding showed that the timing of sivelestat treatment is important. To our knowledge, this is the first study on the optimal timing for the administration of sivelestat.
Although the Japanese research showed elastase inhibition to be beneficial in the pulmonary function of ALI/ARDS patients (38), the STRIVE study did not show that sivelestat was efficacious in a broad spectrum of ALI/ARDS cases (35). The population under study may encompass too broad a range of pathogenetic mechanisms to detect the effectiveness of treatment for specific conditions. This difference in sampling precision may account for the discrepancy in the findings of the STRIVE study and the Japanese report. In several experimental models of SIRS and ARDS, pretreatment with cytokine receptor antagonists or their specific antibodies (39) prevents lung injury. However, these agents have not been demonstrated to be effective in preventing ARDS in clinical trials (40). This discrepancy between experimental and clinical results may be related to the time course of intervention, because the clinical treatment of ARDS usually occurs after the development of SIRS and ARDS. Our study have limitation, we did not measure neutrophil elastase activiy, so inhibition of neutrophil elastase by sivelestat in treatment rats were not shown. Further investigation is required to clarify which patients can benefit from sivelestat and to determine the most effective use of neutrophil elastase inhibitors in the clinical treatment of VILI.
Conclusions
In conclusion, the present study showed that neutrophil elastase might play an important role in the progression of VILI. The mechanisms underlying the effect of neutrophil elastase on lung injury are an increased neutrophil elastase activity, resulting in an increase in pulmonary vascular permeability. Our results indicate that pretreatment with sivelestat can prevent the progression of lung injury caused by neutrophil elastase, although further clinical studies are needed to confirm the results reported in our experimental rat model.
Acknowledgements
This study was supported by the Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital.
Authors’ contributions: All authors contributed conception, analysis, interpretation, revising, and final approval of the manuscript. Do-Hyung Kim and Jae Ho Chung equally served as co-first authors and had full access to all of the data in the study. Sang Gwon Lee took responsibility for the integrity of the data. Yeon Ji Kim carried out experiment and Bong Soo Son provided study management.
Disclosure: The authors declare no conflict of interest.
References
- MacCallum NS, Evans TW. Epidemiology of acute lung injury. Curr Opin Crit Care 2005;11:43-9. [PubMed]
- Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998;338:347-54. [PubMed]
- Dos Santos CC, Slutsky AS. Invited review: mechanisms of ventilator-induced lung injury: a perspective. J Appl Physiol (1985) 2000;89:1645-55. [PubMed]
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334-49. [PubMed]
- Pinhu L, Whitehead T, Evans T, et al. Ventilator-associated lung injury. Lancet 2003;361:332-40. [PubMed]
- Al-Jamal R, Ludwig MS. Changes in proteoglycans and lung tissue mechanics during excessive mechanical ventilation in rats. Am J Physiol Lung Cell Mol Physiol 2001;281:L1078-87. [PubMed]
- Choi G, Wolthuis EK, Bresser P, et al. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents alveolar coagulation in patients without lung injury. Anesthesiology 2006;105:689-95. [PubMed]
- Papaiahgari S, Yerrapureddy A, Reddy SR, et al. Genetic and pharmacologic evidence links oxidative stress to ventilator-induced lung injury in mice. Am J Respir Crit Care Med 2007;176:1222-35. [PubMed]
- Rocker GM, Wiseman MS, Pearson D, et al. Diagnostic criteria for adult respiratory distress syndrome: time for reappraisal. Lancet 1989;1:120-3. [PubMed]
- Russell JA. Management of sepsis. N Engl J Med 2006;355:1699-713. [PubMed]
- Ferring M, Vincent JL. Is outcome from ARDS related to the severity of respiratory failure? Eur Respir J 1997;10:1297-300. [PubMed]
- Kawabata K, Suzuki M, Sugitani M, et al. ONO-5046, a novel inhibitor of human neutrophil elastase. Biochem Biophys Res Commun 1991;177:814-20. [PubMed]
- Takayama M, Ishibashi M, Ishii H, et al. Effects of neutrophil elastase inhibitor (ONO-5046) on lung injury after intestinal ischemia-reperfusion. J Appl Physiol 2001;91:1800-7. [PubMed]
- Inoue Y, Seiyama A, Tanaka H, et al. Protective effects of a selective neutrophil elastase inhibitor (sivelestat) on lipopolysaccharide-induced acute dysfunction of the pulmonary microcirculation. Crit Care Med 2005;33:1814-22. [PubMed]
- Suda K, Kitagawa Y, Ozawa S, et al. Neutrophil elastase inhibitor improves postoperative clinical courses after thoracic esophagectomy. Dis Esophagus 2007;20:478-86. [PubMed]
- Nomura N, Asano M, Saito T, et al. Sivelestat attenuates lung injury in surgery for congenital heart disease with pulmonary hypertension. Ann Thorac Surg 2013;96:2184-91. [PubMed]
- Sakashita A, Nishimura Y, Nishiuma T, et al. Neutrophil elastase inhibitor (sivelestat) attenuates subsequent ventilator-induced lung injury in mice. Eur J Pharmacol 2007;571:62-71. [PubMed]
- Li LF, Huang CC, Lin HC, et al. Unfractionated heparin and enoxaparin reduce high-stretch ventilation augmented lung injury: a prospective, controlled animal experiment. Crit Care 2009;13:R108. [PubMed]
- Quinn DA, Moufarrej RK, Volokhov A, et al. Interactions of lung stretch, hyperoxia, and MIP-2 production in ventilator-induced lung injury. J Appl Physiol 2002;93:517-25. [PubMed]
- Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal Biochem 1985;150:76-85. [PubMed]
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265-75. [PubMed]
- Belperio JA, Keane MP, Burdick MD, et al. Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J Clin Invest 2002;110:1703-16. [PubMed]
- Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 1998;157:294-323. [PubMed]
- Campbell EJ, Campbell MA. Pericellular proteolysis by neutrophils in the presence of proteinase inhibitors: effects of substrate opsonization. J Cell Biol 1988;106:667-76. [PubMed]
- Suttorp N, Nolte A, Wilke A, et al. Human neutrophil elastase increases permeability of cultured pulmonary endothelial cell monolayers. Int J Microcirc Clin Exp 1993;13:187-203. [PubMed]
- Peterson MW, Walter ME, Nygaard SD. Effect of neutrophil mediators on epithelial permeability. Am J Respir Cell Mol Biol 1995;13:719-27. [PubMed]
- Doerschuk CM, Quinlan WM, Doyle NA, et al. The role of P-selectin and ICAM-1 in acute lung injury as determined using blocking antibodies and mutant mice. J Immunol 1996;157:4609-14. [PubMed]
- Sakamaki F, Ishizaka A, Urano T, et al. Effect of a specific neutrophil elastase inhibitor, ONO-5046, on endotoxin-induced acute lung injury. Am J Respir Crit Care Med 1996;153:391-7. [PubMed]
- Kawabata K, Hagio T, Matsuoka S. The role of neutrophil elastase in acute lung injury. Eur J Pharmacol 2002;451:1-10. [PubMed]
- Okayama N, Kakihana Y, Setoguchi D, et al. Clinical effects of a neutrophil elastase inhibitor, sivelestat, in patients with acute respiratory distress syndrome. J Anesth 2006;20:6-10. [PubMed]
- Kubo K, Kobayashi T, Hayano T, et al. Effects of ONO-5046, a specific neutrophil elastase inhibitor, on endotoxin-induced lung injury in sheep. J Appl Physiol 1994;77:1333-40. [PubMed]
- Nishina K, Mikawa K, Takao Y, et al. ONO-5046, an elastase inhibitor, attenuates endotoxin-induced acute lung injury in rabbits. Anesth Analg 1997;84:1097-103. [PubMed]
- Miyazaki Y, Inoue T, Kyi M, et al. Effects of a neutrophil elastase inhibitor (ONO-5046) on acute pulmonary injury induced by tumor necrosis factor alpha (TNFalpha) and activated neutrophils in isolated perfused rabbit lungs. Am J Respir Crit Care Med 1998;157:89-94. [PubMed]
- Tomizawa N, Ohwada S, Ohya T, et al. The effects of a neutrophil elastase inhibitor (ONO-5046.Na) and neutrophil depletion using a granulotrap (G-1) column on lung reperfusion injury in dogs. J Heart Lung Transplant 1999;18:637-45. [PubMed]
- Zeiher BG, Artigas A, Vincent JL, et al. Neutrophil elastase inhibition in acute lung injury: results of the STRIVE study. Crit Care Med 2004;32:1695-702. [PubMed]
- Shasby DM, Vanbenthuysen KM, Tate RM, et al. Granulocytes mediate acute edematous lung injury in rabbits and in isolated rabbit lungs perfused with phorbol myristate acetate: role of oxygen radicals. Am Rev Respir Dis 1982;125:443-7. [PubMed]
- McDonald RJ, Berger EM, Repine JE. Neutrophil-derived oxygen metabolites stimulate thromboxane release, pulmonary artery pressure increases, and weight gains in isolated perfused rat lungs. Am Rev Respir Dis 1987;135:957-9. [PubMed]
- Tamakuma S, Ogawa M, Aikawa N, et al. Relationship between neutrophil elastase and acute lung injury in humans. Pulm Pharmacol Ther 2004;17:271-9. [PubMed]
- Caty MG, Guice KS, Oldham KT, et al. Evidence for tumor necrosis factor-induced pulmonary microvascular injury after intestinal ischemia-reperfusion injury. Ann Surg 1990;212:694-700. [PubMed]
- Kollef MH, Schuster DP. The acute respiratory distress syndrome. N Engl J Med 1995;332:27-37. [PubMed]