
The complete blood count to diagnose septic shock
The use of blood count to diagnose septic shock
The complete blood count has long been an integral component of diagnosing septic shock. For example, the initial definition of systemic inflammatory response syndrome (SIRS) in 1992 included abnormality in white blood cell count (either elevated or reduced) or a normal white blood cell count with >10% bands (1). Ironically, these two parameters, which attract the most attention [white blood count (WBC) and bandemia], might be among the less useful components of the complete blood count.
A complete blood count can provide a wealth of information, much of which may not immediately be obvious. Understanding how to fully interpret this test is beneficial for several reasons. Most patients undergoing evaluation will already have a complete blood count ordered, so this information is often immediately available. Interpreting the nuances of this test may provide additional diagnostic information at no additional cost. Finally, the complete blood count is a ubiquitous test which should be available nearly anywhere.
Our goal will be to extract as much information from blood count as possible. Given that patients will usually already have a blood count obtained, it is entirely acceptable to pay attention to components of the blood count which have large grey zones (ranges within which no useful information is provided). This is unlike most laboratory tests, wherein the yield of the test must be weighed against the test’s cost.
WBC
The WBC is the most commonly used metric to investigate infection, but is also the least useful. Septic shock may cause either leukocytosis or leukopenia. Many septic patients exist between these two extremes, with a normal WBC (such patients often develop leukocytosis in a delayed fashion). For example, half of patients presenting to the hospital with bacteremia may have a normal WBC (2). Thus, while a substantially abnormal WBC may suggest the presence of infection, a normal WBC reveals little.
If the WBC is extremely low, then determination of the absolute neutrophil count must be made (the absolute number of mature neutrophils plus bands present). Neutropenia is generally defined as an absolute neutrophil count below 500/microliter, or a count in the range of 500–1,000/microliter which is decreasing. Patients with neutropenia often fail to manifest focal signs of infection. There must be a high index of suspicion for infection in patients with neutropenia (for example, the mere presence of fever generally indicates the need for broad-spectrum antibiotics).
Left shift
Infection stimulates the production of cytokines which trigger the release of immature granulocytes from the bone marrow (e.g., granulocyte colony stimulating factor). This is reflected by the presence of immature cells in peripheral circulation. The least immature cells commonly seen in peripheral circulation are bands. With increasing cytokine levels, progressively more immature cells may be released as well (including promyelocytes, metamyelocytes, and myelocytes).
An important drawback of left shift is that release of immature cells from the bone marrow is often delayed, emerging about one day after clinical infection. This can cause a left shift to be absent when a patient first presents with septic shock.
Band count
Traditionally, left-shifting has been determined by the presence of band neutrophils in the blood. This is assessed using a manual cell count (usually based on the number of bands found within one hundred leukocytes).
Measurement of bandemia has two unique drawbacks. First, a manual cell count is required, which introduces a considerable delay to the availability of these results (3). Given the urgency of reaching an accurate diagnosis of septic shock, a delay of even a few hours may be very problematic (4). Second, measurement of bands is subject to inter-observer and inter-hospital variability, due to confusion in the literature regarding exactly how to define bands (5).
Bandemia has a low sensitivity for infection, but a reasonably high specificity (~85% using a cutoff of >10% bands) (5-7). Other potential causes of bandemia may include surgery, hemorrhage, tissue necrosis, myeloproliferative disorders, and exogenous granulocyte cell stimulating factor. Thus, if a substantial bandemia is discovered, it should be regarded as potential evidence of sepsis until demonstrated otherwise.
Immature granulocytes
This term refers to the percent of circulating leukocytes which are promyelocytes, myelocytes, or metamyelocytes. These represent more immature forms than bands, so they are generally present at lower levels than bands.
Modern hematology analyzers are capable of automatically counting the percent of immature granulocytes among thousands of cells, as a component of a routine complete blood count. This has promise for yielding a precise, generalizable, and rapid measurement of left shift.
Unfortunately, the current literature regarding immature granulocytes is limited by considerable heterogeneity. Some investigators have found immature granulocytes to have diagnostic value, whereas others have found the test to be nearly worthless (8-11). Studies are divided between using the absolute number of immature granulocytes versus the percentage of immature granulocytes (with no clear evidence regarding which might be superior) (12,13). The optimal cutoff value ranges widely between studies, from 0.2% to 3% (4,6,14,15).
Currently, the utility of immature granulocyte count mirrors that of the band count. Markedly elevated values suggest infection (e.g., >3%). Clinicians should be aware that immature granulocyte measurements have supplanted band counts in many modern automated hemograms. With increased attention to this parameter, practitioners may gain an appreciation for the normal range within their patient population. Hopefully, future research will clarify the optimal cutoff values and provide further validation for this measurement.
Neutrophil to lymphocyte ratio (NLR)
The NLR is simply the ratio of neutrophils/lymphocytes. This is easily calculated from any differential cell count (as either the ratio of absolute cell counts, or as the ratio of relative cell counts). Physiologic stress generally increases the number of neutrophils and decreases the number of lymphocytes, so it will drive up the NLR. The precise mechanism of NLR elevation is unclear, but likely involves some combination of endogenous cortisol and catecholamines (both of which are known to increase neutrophil counts while decreasing lymphocyte counts) (16,17). Sepsis also stimulates lymphocyte apoptosis, so septic shock may cause particularly dramatic elevation of NLR, compared to other forms of physiologic stress (18).
NLR is not an indication solely of inflammation, but rather it may be increased by any source of physiologic stress (e.g., hypovolemic shock). Consequently, NLR may be useful in sorting out patients with severe systemic illness versus patients with milder illness (i.e., “sick versus not sick”). NLR is not helpful in differentiating the precise cause of the patient’s illness (e.g., sterile pancreatitis vs. ascending cholangitis).
NLR increases rapidly following acute physiologic stress, often within 6 hours (19). This prompt rise can make NLR a superior reflection of acute illness, compared to other components of the complete blood count which usually take longer to increase (e.g., WBC and left shift) (6). In many patients with early septic shock, the NLR may be the only parameter which accurately reflects the severity of illness.
Unlike immature granulocytes, the NLR is quantified in a uniform fashion across all medical literature. Furthermore, distinguishing neutrophils from lymphocytes is straightforward, so the measured NLR value should be uniform regardless of which hemocytometer is used.
Some limitations must be borne in mind when interpreting the NLR (20). Exogenous steroids can increase the NLR, whereas adrenal insufficiency may decrease it. The use of NLR hasn’t been validated among patients with human immunodeficiency virus (HIV) or active hematologic disorders (e.g., leukemia, chemotherapy).
Ljungström et al. performed a prospective study of 1,572 patients presenting to the emergency department with a suspicion of sepsis (21). For the diagnosis of severe sepsis or septic shock, NLR was superior to C-reactive protein and equivalent to procalcitonin (in terms of the area under the receiver-operator curve). With a low cutoff of NLR >3, the test was 96% sensitive but only 10% specific. Raising the cutoff value to NLR >10 improved the specificity to 56%, but at the cost of lowering the sensitivity to 80%.
NLR is less accurate at identification of septic shock within a population of critically ill patients, all of whom have been admitted to an intensive care unit. Patients with non-infectious critical illness tend to have moderately elevated NLR, so NLR is less adept at detecting sepsis within this context. One study of 664 patients in an intensive care unit (ICU) found NLR >10 to have a sensitivity of 66% and specificity of 53% for sepsis (22). Another study of 452 ICU patients used a lower NLR cutoff (>5) to achieve a higher sensitivity (81%), but at the cost of lower specificity (36%).
Although limited literature exists regarding NLR for the diagnosis of septic shock, a large body of literature has evaluated NLR for the diagnosis of bacteremia or various focal infections (e.g., appendicitis). Some themes run through these articles. First, NLR is universally superior to the WBC (23,24). This should come as no surprise, given that infection will tend to increase NLR, whereas infection may either increase or decrease the WBC. Second, NLR often has similar or better performance compared to C-reactive protein (but is usually inferior to procalcitonin) (25).
NLR may also be used to track the clinical course of septic shock. With successful therapy, NLR will generally begin to fall within a few days (26). Failure of the NLR to improve over time correlates with poor prognosis.
Clinical interpretation of the NLR value
A normal NLR is about 1–3 (27,28). Values increase in proportion to the degree of physiologic stress, especially in septic shock.
Gürol et al. compared procalcitonin with other parameters among 1,468 patients with suspected infection (Table 1) (29). Although procalcitonin isn’t a perfect index of septic shock, this comparison helps calibrate our interpretation of NLR. This study found NLR to correlate more strongly with procalcitonin than either WBC or C-reactive protein.
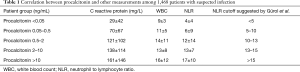
Full table
The NLR should be interpreted within clinical context (including consideration of other known sources of physiologic stress which the patient is under). For example, a patient with diabetic ketoacidosis and hypovolemic shock could have an elevated NLR simply due to these stressors, so an NLR of 14 in that context shouldn’t be alarming. Alternatively, an NLR of 14 in a patient with cellulitis and no other apparent source of physiologic stress should raise a red flag for the presence of systemic inflammation (e.g., toxic shock syndrome).
Ideally, clinicians should always consider the NLR when evaluating a complete blood count (not only when there is concern for sepsis). Over time, this will foster a nuanced appreciation of how the test performs within their clinical context. A rough guide to the interpretation of NLR in patients with features of infection and concern for septic shock is shown in Figure 1. Some reasonable benchmarks might be three and ten.
- Normal NLR (<3): 90–95% of patients with severe sepsis have an NLR above 3. Therefore, NLR values below three don’t absolutely exclude septic shock, but they should direct attention to other diagnostic possibilities. For critically ill patients with an NLR which seems disproportionately low compared to their illness severity, adrenal insufficiency might also be considered (since lack of cortisol response to stress could prevent the NLR from increasing).
- Grey zone (NLR of roughly 3–10): values in this range provide no clear guidance about the presence or absence of septic shock. Since NLR is freely available in all patients, it’s acceptable for it to have this sizable grey zone.
- Considerably elevated NLR (>10): this suggests the presence of severe systemic stress (as could be caused by septic shock or another critical illness). A cutoff value of >10 is only weakly specific for sepsis (~65%) (2,4,15,30). Thus, a value slightly above ten isn’t diagnostic, but instead is merely a clue to investigate further (31). Ten is a convenient cutoff value, because it can easily be determined whether the NLR is above or below ten without a calculator (by mentally shifting the decimal point in the lymphocyte count and comparing this to the neutrophil count). The higher the NLR value is above ten, the more specific and concerning it becomes.

Platelet count
Like WBC, platelet count may be increased or decreased by infection. Platelet count is an acute phase reactant, so platelets are often elevated in chronic, smoldering infection. Septic shock commonly causes platelet consumption, so thrombocytopenia is more commonly seen here (in some cases, evolving into full-blown disseminated intravascular coagulation).
Thrombocytopenia can be a useful clue to the diagnosis of sepsis. It is present in ~40% of patients with septic shock. The severity of thrombocytopenia is a strong prognostic factor for mortality. However, this is a relatively nonspecific finding, as thrombocytopenia is commonly found among critically ill patients. Thus, thrombocytopenia may function as a red flag suggesting the presence of severe systemic illness, without revealing any precise etiology. Chronic thrombocytopenia is a common feature of many illnesses (e.g., cirrhosis), so acute thrombocytopenia is more worrisome than chronic thrombocytopenia.
Emerging parameters
With ongoing improvements in hemocytometer technology, new parameters are likely to emerge. For example, monocyte distribution width appears particularly promising. Two studies have demonstrated that this may identify septic patients in the emergency department with good accuracy (area under the receiver-operator curve of ~0.75) (32,33). A challenge facing more sophisticated parameters will be ensuring that they translate accurately across different hemocytometer manufacturers. An additional challenge is that more sophisticated parameters (such as monocyte distribution width) are often not reported to clinicians.
Conclusions
The complete blood cell count with differential may contain a considerable amount of information, often providing early clues to the diagnosis of septic shock. Unfortunately, typical practice is to overlook most of this data, focusing largely on the least useful parameter (the white blood cell count).
The single most useful parameter from the blood count might be the NLR, given its early responsiveness to infection and unidirectional response to physiologic stress (unlike the white blood cell count, which may either rise or fall in septic shock). However, like all elements of the blood count, this too is nonspecific (potentially alerting the clinician that the patient is systemically unwell, without necessarily proving the presence of septic shock).
Acknowledgments
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644-55. [Crossref] [PubMed]
- Seigel TA, Cocchi MN, Salciccioli J, et al. Inadequacy of temperature and white blood cell count in predicting bacteremia in patients with suspected infection. J Emerg Med 2012;42:254-9. [Crossref] [PubMed]
- Martins EC, Silveira LDF, Viegas K, et al. Neutrophil-lymphocyte ratio in the early diagnosis of sepsis in an intensive care unit: a case-control study. Rev Bras Ter Intensiva 2019;31:64-70. [Crossref] [PubMed]
- Davis S, Shesser R, Authelet K, et al. "Bandemia" without leukocytosis: A potential Emergency Department diagnostic pitfall. Am J Emerg Med 2019;37:1970-1971. [Crossref] [PubMed]
- Cornbleet PJ. Clinical utility of the band count. Clin Lab Med 2002;22:101-36. [Crossref] [PubMed]
- Honda T, Uehara T, Matsumoto G, et al. Neutrophil left shift and white blood cell count as markers of bacterial infection. Clin Chim Acta 2016;457:46-53. [Crossref] [PubMed]
- Cavallazzi R, Bennin CL, Hirani A, et al. Is the band count useful in the diagnosis of infection? An accuracy study in critically ill patients. J Intensive Care Med 2010;25:353-7. [Crossref] [PubMed]
- Porizka M, Volny L, Kopecky P, et al. Immature granulocytes as a sepsis predictor in patients undergoing cardiac surgery. Interact Cardiovasc Thorac Surg 2019;28:845-51. [Crossref] [PubMed]
- Karon BS, Tolan NV, Wockenfus AM, et al. Evaluation of lactate, white blood cell count, neutrophil count, procalcitonin and immature granulocyte count as biomarkers for sepsis in emergency department patients. Clin Biochem 2017;50:956-8. [Crossref] [PubMed]
- Ayres LS, Sgnaolin V, Munhoz TP. Immature granulocytes index as early marker of sepsis. Int J Lab Hematol 2019;41:392-6. [Crossref] [PubMed]
- Ansari-Lari MA, Kickler TS, Borowitz MJ. Immature granulocyte measurement using the Sysmex XE-2100. Relationship to infection and sepsis. Am J Clin Pathol 2003;120:795-9. [Crossref] [PubMed]
- Buoro S, Mecca T, Azzarà G, et al. Extended leukocyte differential count and C-reactive protein in septic patients with liver impairment: diagnostic approach to evaluate sepsis in intensive care unit. Ann Transl Med 2015;3:244. [PubMed]
- Arneth BM, Ragaller M, Hommel K, et al. Novel parameters of extended complete blood cell count under fluorescence flow cytometry in patients with sepsis. J Clin Lab Anal 2014;28:130-5. [Crossref] [PubMed]
- van der Geest PJ, Mohseni M, Brouwer R, et al. Immature granulocytes predict microbial infection and its adverse sequelae in the intensive care unit. J Crit Care 2014;29:523-7. [Crossref] [PubMed]
- Bernstein LH, Rucinski J. Measurement of granulocyte maturation may improve the early diagnosis of the septic state. Clin Chem Lab Med 2011;49:2089-95. [Crossref] [PubMed]
- Benschop RJ, Rodriguez-Feuerhahn M, Schedlowski M. Catecholamine-induced leukocytosis: early observations, current research, and future directions. Brain Behav Immun 1996;10:77-91. [Crossref] [PubMed]
- Onsrud M, Thorsby E. Influence of in vivo hydrocortisone on some human blood lymphocyte subpopulations. I. Effect on natural killer cell activity. Scand J Immunol 1981;13:573-9. [Crossref] [PubMed]
- Zhang Y, Li J, Lou J, et al. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit Care 2011;15:R70. [Crossref] [PubMed]
- Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy 2001;102:5-14. [PubMed]
- Karakonstantis S, Kalemaki D, Tzagkarakis E, et al. Pitfalls in studies of eosinopenia and neutrophil-to-lymphocyte count ratio. Infect Dis (Lond) 2018;50:163-74. [Crossref] [PubMed]
- Ljungström L, Pernestig AK, Jacobsson G, et al. Diagnostic accuracy of procalcitonin, neutrophil-lymphocyte count ratio, C-reactive protein, and lactate in patients with suspected bacterial sepsis. PLoS One 2017;12:e0181704. [Crossref] [PubMed]
- Westerdijk K, Simons KS, Zegers M, et al. The value of the neutrophil-lymphocyte count ratio in the diagnosis of sepsis in patients admitted to the Intensive Care Unit: a retrospective cohort study. PLoS One 2019;14:e0212861. [Crossref] [PubMed]
- Orfanu AE, Popescu C, Leuștean A, et al. The importance of haemogram parameters in the diagnosis and prognosis of septic patients. J Crit Care Med (Targu Mures) 2017;3:105-10. [Crossref] [PubMed]
- Goodman DA, Goodman CB, Monk JS. Use of the neutrophil:lymphocyte ratio in the diagnosis of appendicitis. Am Surg 1995;61:257-9. [PubMed]
- de Jager CP, van Wijk PT, Mathoera RB, et al. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit Care 2010;14:R192. [Crossref] [PubMed]
- Terradas R, Grau S, Blanch J, et al. Eosinophil count and neutrophil-lymphocyte count ratio as prognostic markers in patients with bacteremia: a retrospective cohort study. PLoS One 2012;7:e42860. [Crossref] [PubMed]
- Lee JS, Kim NY, Na SH, et al. Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine (Baltimore) 2018;97:e11138. [Crossref] [PubMed]
- Holub M, Beran O, Kaspříková N, et al. Neutrophil to lymphocyte count ratio as a biomarker of bacterial infections. Cent Eur J Med 2012;7:258-61.
- Gürol G, Çiftci İH, Terizi HA, et al. Are there standardized cutoff values for neutrophil-lymphocyte ratios in bacteremia or sepsis? J Microbiol Biotechnol 2015;25:521-5. [Crossref] [PubMed]
- Zhang HB, Chen J, Lan QF, et al. Diagnostic values of red cell distribution width, platelet distribution width and neutrophil-lymphocyte count ratio for sepsis. Exp Ther Med 2016;12:2215-9. [Crossref] [PubMed]
- Marik PE. Don't miss the diagnosis of sepsis! Crit Care 2014;18:529. [Crossref] [PubMed]
- Crouser ED, Parrillo JE, Seymour C, et al. Improved early detection of sepsis in the ed with a novel monocyte distribution width biomarker. Chest 2017;152:518-26. [Crossref] [PubMed]
- Crouser ED, Parrillo JE, Seymour CW, et al. Monocyte distribution width: a novel indicator of sepsis-2 and sepsis-3 in high-risk emergency department patients. Crit Care Med 2019;47:1018-25. [Crossref] [PubMed]


