
Early norepinephrine use in septic shock
Introduction
Septic shock, defined as sepsis with concurrent hypotension requiring vasopressor therapy and hyperlactatemia, is a common challenge in the intensive care unit (ICU) (1). Despite multiple therapeutic advances over the past decades, it remains the leading cause of morbidity and mortality in critically ill patients, accounting for about 30% to 40% of ICU mortality (2).
Septic shock is characterized by hypovolemia (both relative and absolute) and decreased vascular tone, which contributes much to the severity of hypotension. Physiologically, organ blood flow depends on perfusion pressure when mean arterial pressure (MAP) decreases below a certain critical value. Thus, at the early phase, both fluid resuscitation aiming to correct hypovolemia (3) and vasopressors—mainly norepinephrine (NE) as a first-line agent (3)—aiming to restore the vascular tone are important to ensure organ perfusion (4,5). Nevertheless, a large amount of fluids will inevitably increase the risk of fluid overload, which is a common complication during septic shock resuscitation (6). Besides, after the very early phase, only half of the patients are fluid-responders (7), which means that fluid administration cannot increase further cardiac output (CO). Furthermore, it has been shown that early (within a median time interval of 1.3 hours after admission in the ICU) and exclusive administration of NE restored an adequate MAP within a relatively a short period of time (30 min) in all patients and was associated with a survival rate, which compared favorably with that predicted by illness severity scores and with that reported in the literature for similar patients (8). However, the timing to start NE is crucial. Recently, a group of 34 members of the European Society of Intensive Care Medicine (ESICM) considered as experts in hemodynamics have formulated recommendations on the use of vasopressors in shock states. One of the recommendations was to administer NE in the initial phase of septic shock even when hypovolemia is not completely corrected by fluid administration (9). This recommendation was based on a “reasonable” consensus meaning that between 70% and 80% of the experts agreed on it. This review aims at providing arguments in favor of an early administration of NE in septic shock (Table 1).
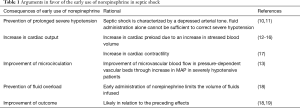
Full table
Early administration of a vasopressor would prevent prolonged severe hypotension
It is now well demonstrated that not only the degree but also the duration of hypotension, especially in the first 48 h, is crucial for patient outcome (10,11). As septic shock is characterized by a depressed arterial tone, it is clear that fluid administration alone cannot be sufficient to correct severe hypotension. Early correction of hypotension with a potent vasopressor such as NE should thus shorten the duration of hypotension compared to fluid alone.
Early administration of norepinephrine increases cardiac output in septic shock
NE is an α1-adrenergic agent with β1-adrenergic properties. Clinical studies showed that NE can increase CO when initiated early in septic shock (12,13,17). In a series of 105 severely hypotensive patients with septic shock, we found that early administration of NE aimed at rapidly achieving a sufficient MAP was able to increase stroke volume and CO (12). The decision to initiate norepinephrine was made on the basis of a low diastolic arterial pressure (DAP)—considered as a marker of a low arterial tone (20)—and not after that fluid resuscitation had been fully completed. The increase in stroke volume was associated with an increased global end-diastolic volume—a marker of cardiac preload—and a reduced pulse pressure variation (PPV), which is a marker of preload responsiveness (12). Similar results were found in patients with septic shock and preload responsiveness defined by a positive passive leg raising test (14). Taken together, these results suggest that NE through its α1-adrenergic mediated effects is able to increase cardiac preload and CO in patients with preload responsiveness, a condition which is quite common in early sepsis. It has been postulated that NE is able to redistribute venous blood from the unstressed to the stressed blood volume as confirmed by studies showing an increase in mean systemic filling pressure with NE in septic patients (15) and in post-cardiac surgery patients (16). Unlike recent clinical studies (12,13,17), studies performed in the past century failed to show an increase in CO with NE (21-23), probably because large amount of fluids were administered before NE initiation resulting in high CO values and thus in low cardiac preload reserve.
Furthermore, NE may increase stroke volume by increasing cardiac contractility. This was recently illustrated in a study that included 38 patients with septic shock who had been resuscitated for less than three hours and in whom MAP remained lower than 65 mmHg. Echocardiographic variables were obtained before and after increasing the dose of NE in order to increase MAP to at least 65 mmHg (17). Interestingly, we observed an improvement in left and right ventricle systolic function variables and an increase in CO. These results were also found in the subgroup of patients with low left ventricular ejection fraction (LVEF) (17). The significant increase in LVEF with NE, in spite of the increased left ventricular afterload strongly suggests that NE increased the left ventricular contractility. The two potential mechanisms for such a beneficial effect of NE could be an improved left ventricular perfusion in relation to the increased DAP and the left ventricular consequence of β1-adrenergic receptor stimulation (17). Note that experimental data suggests that this latter effect is time-dependent with an initial phase of potentiation of the β1-adrenergic response (‘upregulation’) followed by a downregulation phase (24). Therefore, NE might increase the cardiac contractility through β1-adrenergic stimulation mainly in the early phase of septic shock.
Early initiation of norepinephrine may improve microcirculation in severe septic shock
It is generally assumed that septic shock is associated with impaired microcirculation even in patients with preserved or corrected macrocirculation (25). However, in severely hypotensive patients, an improvement of microvascular blood flow could be expected with correction of hypotension due to correction of low organ perfusion pressure. Georger et al. investigated the effects of early administration of NE on microcirculation assessed using near-infrared spectroscopy at the level of thenar eminence in patients with septic shock (13). With NE, the MAP significantly increased from 54 to 77 mmHg and tissue oxygen saturation (StO2) significantly increased from 75% to 78% (normal values are around 82%) (13). Vascular occlusion tests were performed to assess the hyperemic response to the local hypoxic stimulus created by transient ischemia of the occluded vascular bed. Increasing MAP with NE resulted in a significant increase in the StO2 recovery slope (13). This result is of interest since the StO2 recovery slope, which reflects the capacity of microvessels to be recruited in response to local hypoxia, was previously demonstrated to be a prognostic factor in patients with septic shock (26). It could be postulated that increasing the MAP in severely hypotensive patients improved microvascular blood flow in pressure-dependent vascular beds and hence improved muscle tissue oxygenation and microcirculatory recruitment capacities. In accordance with these results, previous data showed a good correlation between the MAP and sublingual microcirculatory indices in the first six hours of septic shock resuscitation (27). All these findings strongly suggest that impairment of microcirculation due to a potential NE-induced excessive vasoconstriction is not a reality when MAP is low. Even when MAP is already at 65 mmHg, clinical studies showed that increasing MAP up to 85 or 90 mmHg did not impair microcirculation (28) but may even improve it (29-31).
Early initiation of norepinephrine prevents harmful fluid overload
It is now well-established that positive fluid balance is independently associated to increased mortality rate in septic shock (32-34). The mechanisms by which excessive fluid administration may worsen outcome are multiple. They include peripheral tissue edema with risks of multiple organ dysfunction, pulmonary edema with risks of profound hypoxemia, degradation of endothelial glycocalyx (35) with risks of increased vascular permeability, marked increase in venous pressures—especially in the case of preload unresponsiveness—with risks of decrease in organ perfusion pressure (36), and hemodilution (37). It could thus be tempting to restrict fluid administration even at the initial stage of resuscitation by starting vasopressors early. In this regard, in a retrospective study in septic shock patients, those in whom NE was administered within the first two hours of resuscitation received less fluid than those who received a delayed NE administration (18). Nevertheless, starting NE early to counteract the sepsis-induced depression of vasomotor tone does not imply discontinuation of fluid infusion, in particular if signs of profound hypovolemia and/or evident fluid losses are present.
Early norepinephrine administration may improve outcome
A retrospective study conducted in a cohort of 213 patients with septic shock showed the time to initiate NE was an independent factor associated with mortality (18). This strongly suggested that the later NE was initiated, the worse the outcome. Interestingly, in the subgroup of patients who received NE early, the duration of hypotension and of NE administration were shorter and the total dose of NE was lower than in the subgroup of patients who received NE late (18). This indicates that administering NE early does not necessarily result in increased catecholamine load. A recent single-center randomized double-blind placebo-controlled trial compared two strategies in septic shock (19): one subgroup of patients received NE early (n=155) while in the other subgroup initiation of NE was delayed (n=155). The primary outcome was shock control rate by six hours after diagnosis. The shock control was defined as: MAP ≥65 mmHg with either urine flow ≥0.5 mL/kg/hour for two consecutive hours or decreased serum lactate ≥10% from baseline. According to that definition, shock was controlled in 76% of patients in the early NE group vs. 48% of patients in the control group (P<0.001) (19). There was not any difference in the mortality rate, which was not the primary endpoint of the trial, but there were lower rates of cardiogenic pulmonary edema and new episodes of cardiac arrhythmia in the early NE group compared to the control group. The authors concluded that their findings support the benefit of early administration of NE in septic shock (19).
How to identify patients who need very early initiation of norepinephrine?
The latest version of the Surviving Sepsis Campaign (SSC) guidelines suggested that vasopressors should be administered only after the initial fluid resuscitation (30 mL/kg of crystalloids within the first 3 h) (3). The updated publication of the SSC bundles proposed a new 1-hour bundle (4), indicating that vasopressors are recommended if the patient is hypotensive during or after fluid resuscitation to keep MAP ≥65 mmHg. Although this last recommendation (4) is a significant advance as compared to the SSC guidelines (3), it remains unclear and does not provide clinicians with clues to identify patients who need NE urgently. Sepsis is not a disease but a syndrome covering various host responses to infection. In addition, the location of the infection also contributes to differences in pathophysiologic and clinical patterns. For example, sepsis of abdominal origin is more often associated with profound hypovolemia than sepsis of lung origin. In some patients, hypovolemia is the predominant mechanism responsible for the hemodynamic failure, in some others vascular tone depression or myocardial depression are the predominant mechanisms. As there is no unique hemodynamic pattern, the therapeutic approach must be personalized. Clearly, NE should be administered when the vascular tone depression is judged to contribute significantly to hypotension. The DAP could be used for that purpose as it reflects the vascular tone (38). Thus, a low DAP is mainly due to depressed vascular tone (20,38). Since tachycardia elevates DAP due to the short diastolic time, a low value of DAP (e.g., <40 mmHg) is strongly suggestive of a markedly depressed arterial tone and should prompt initiation of NE urgently. This was clearly indicated in the 2012 version of the SSC guidelines (39) but disappeared inexplicably in the last version (3).
Conclusions
Early administration of NE during septic shock may benefit to patients by several ways: an increase in cardiac output due to increases in cardiac preload and contractility, an improvement in microcirculation and a limitation of fluid overload. In addition, there are recent data suggesting that early administration of NE may improve outcome. A low DAP—as a marker of depressed vascular tone—can simply identify septic patients that need NE urgently.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:775-87. [Crossref] [PubMed]
- Vincent JL, Jones G, David S, et al. Frequency and mortality of septic shock in Europe and North America: a systematic review and meta-analysis. Crit Care 2019;23:196. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 Update. Intensive Care Med 2018;44:925-8. [Crossref] [PubMed]
- De Backer D, Orbegozo Cortes D, Donadello K, et al. Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence 2014;5:73-9. [Crossref] [PubMed]
- Kelm DJ, Perrin JT, Cartin-Ceba R, et al. Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock 2015;43:68-73. [Crossref] [PubMed]
- Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest 2002;121:2000-8. [Crossref] [PubMed]
- Morimatsu H, Singh K, Uchino S, et al. Early and exclusive use of norepinephrine in septic shock. Resuscitation 2004;62:249-54. [Crossref] [PubMed]
- Scheeren TWL, Bakker J, De Backer D, et al. Current use of vasopressors in septic shock. Ann Intensive Care 2019;9:20. [Crossref] [PubMed]
- Varpula M, Tallgren M, Saukkonen K, et al. Hemodynamic variables related to outcome in septic shock. Intensive Care Med 2005;31:1066-71. [Crossref] [PubMed]
- Dünser MW, Ruokonen E, Pettila V, et al. Association of arterial blood pressure and vasopressor load with septic shock mortality: a post hoc analysis of a multicenter trial. Crit Care 2009;13:R181. [Crossref] [PubMed]
- Hamzaoui O, Georger JF, Monnet X, et al. Early administration of norepinephrine increases cardiac preload and cardiac output in septic patients with life-threatening hypotension. Crit Care 2010;14:R142. [Crossref] [PubMed]
- Georger JF, Hamzaoui O, Chaari A, et al. Restoring arterial pressure with norepinephrine improves muscle tissue oxygenation assessed by near-infrared spectroscopy in severely hypotensive septic patients. Intensive Care Med 2010;36:1882-9. [Crossref] [PubMed]
- Monnet X, Jabot J, Maizel J, et al. Norepinephrine increases cardiac preload and reduces preload dependency assessed by passive leg raising in septic shock patients. Crit Care Med 2011;39:689-94. [Crossref] [PubMed]
- Persichini R, Silva S, Teboul JL, et al. Effects of norepinephrine on mean systemic pressure and venous return in human septic shock. Crit Care Med 2012;40:3146-53. [Crossref] [PubMed]
- Maas JJ, Pinsky MR, de Wilde RB, et al. Cardiac output response to norepinephrine in postoperative cardiac surgery patients: interpretation with venous return and cardiac function curves. Crit Care Med 2013;41:143-50. [Crossref] [PubMed]
- Hamzaoui O, Jozwiak M, Geffriaud T, et al. Norepinephrine exerts an inotropic effect during the early phase of human septic shock. Br J Anaesth 2018;120:517-24. [Crossref] [PubMed]
- Bai X, Yu W, Ji W, et al. Early versus delayed administration of norepinephrine in patients with septic shock. Crit Care 2014;18:532. [Crossref] [PubMed]
- Permpikul C, Tongyoo S, Viarasilpa T, et al. Early Use of Norepinephrine in Septic Shock Resuscitation (CENSER). A Randomized Trial. Am J Respir Crit Care Med 2019;199:1097-105. [Crossref] [PubMed]
- Hamzaoui O, Teboul JL. Importance of diastolic arterial pressure in septic shock: PRO. J Crit Care 2019;51:238-40. [Crossref] [PubMed]
- Desjars P, Pinaud M, Potel G, et al. A reappraisal of norepinephrine therapy in human septic shock. Crit Care Med 1987;15:134-7. [Crossref] [PubMed]
- Martin C, Papazian L, Perrin G, et al. Norepinephrine or dopamine for the treatment of hyperdynamic septic shock? Chest 1993;103:1826-31. [Crossref] [PubMed]
- Martin C, Viviand X, Arnaud S, et al. Effects of norepinephrine plus dobutamine or norepinephrine alone on left ventricular performance of septic shock patients. Crit Care Med 1999;27:1708-13. [Crossref] [PubMed]
- Abi-Gerges N, Tavernier B, Mebazaa A, et al. Sequential changes in autonomic regulation of cardiac myocytes after in vivo endotoxin injection in rat. Am J Respir Crit Care Med 1999;160:1196-204. [Crossref] [PubMed]
- De Backer D, Creteur J, Preiser JC, et al. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med 2002;166:98-104. [Crossref] [PubMed]
- Creteur J, Carollo T, Soldati G, et al. The prognostic value of muscle StO2 in septic patients. Intensive Care Med 2007;33:1549-56. [Crossref] [PubMed]
- Trzeciak S, Dellinger RP, Parrillo JE, et al. Microcirculatory Alterations in Resuscitation and Shock Investigators. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med 2007;49:88-98. [Crossref] [PubMed]
- Dubin A, Pozo MO, Casabella CA, et al. Increasing arterial blood pressure with norepinephrine does not improve microcirculatory blood flow: a prospective study. Crit Care 2009;13:R92. [Crossref] [PubMed]
- Thooft A, Favory R, Salgado DR, et al. Effects of changes in arterial pressure on organ perfusion during septic shock. Crit Care 2011;15:R222. [Crossref] [PubMed]
- Kazune S, Caica A, Luksevics E, et al. Impact of increased mean arterial pressure on skin microcirculatory oxygenation in vasopressor-requiring septic patients: an interventional study. Ann Intensive Care 2019;9:97. [Crossref] [PubMed]
- Fiorese Coimbra KT, de Freitas FGR, Bafi AT, et al. Effect of Increasing Blood Pressure With Noradrenaline on the Microcirculation of Patients With Septic Shock and Previous Arterial Hypertension. Crit Care Med 2019;47:1033-40. [Crossref] [PubMed]
- Boyd JH, Forbes J, Nakada TA, et al. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 2011;39:259-65. [Crossref] [PubMed]
- Sakr Y, Rubatto Birri PN, Kotfis K, et al. Intensive Care Over Nations Investigators. Higher Fluid Balance Increases the Risk of Death From Sepsis: Results From a Large International Audit. Crit Care Med 2017;45:386-94. [Crossref] [PubMed]
- Marik PE, Linde-Zwirble WT, Bittner EA, et al. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Med 2017;43:625-32. [Crossref] [PubMed]
- Hippensteel JA, Uchimido R, Tyler PD, et al. Intravenous fluid resuscitation is associated with septic endothelial glycocalyx degradation. Crit Care. 2019;23:259. [Crossref] [PubMed]
- Marik PE. Iatrogenic salt water drowning and the hazards of a high central venous pressure. Ann Intensive Care 2014;4:21. [Crossref] [PubMed]
- Monnet X, Julien F, Ait-Hamou N, et al. Lactate and venoarterial carbon dioxide difference/arterial-venous oxygen difference ratio, but not central venous oxygen saturation, predict increase in oxygen consumption in fluid responders. Crit Care Med 2013;41:1412-20. [Crossref] [PubMed]
- Lamia B, Chemla D, Richard C, et al. Clinical review: interpretation of arterial pressure wave in shock states. Crit Care 2005;9:601-6. [Crossref] [PubMed]
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165-228. [Crossref] [PubMed]


