
Sepsis trends: increasing incidence and decreasing mortality, or changing denominator?
Many epidemiologic studies have reported that the incidence of sepsis has dramatically increased over the past several decades while mortality rates have fallen (1-5). Despite improvements in sepsis outcomes, these reports indicate that the total burden of sepsis-associated mortality has increased owing to the marked rise in the number of sepsis cases. Commonly cited explanations for the increase in sepsis incidence include an aging population with more predisposing comorbidities, more frequent use of immunosuppression, more invasive procedures and medical devices, and the spread of multi-drug resistant pathogens (6-8). Declining sepsis-associated mortality rates are generally attributed to better sepsis recognition, faster treatment, increasing uptake of sepsis bundles, and general improvements in the care of critically ill patients (9-13).
However, there is considerable controversy over the degree to which these observed trends represent true increases in disease incidence and improvements in outcomes versus artifacts of changing sepsis diagnosis and coding practices over time (14,15). Specifically, critics have raised the concern that these reports could be due to the “Will Rogers phenomenon”, or ascertainment bias from the introduction of new diagnostic strategies that lead to improved detection and labeling of patients with sepsis, particularly those with milder disease (16). This then produces a misleading impression of decreases in mortality that do not reflect actual improvements in patient outcomes. Sepsis is highly susceptible to this bias due to widespread, aggressive, and successful efforts to improve sepsis awareness, enhance sepsis recognition and management, and improve sepsis coding. Patients that were once labeled as “infection” with concurrent “organ dysfunction” are now more likely to be diagnosed with “severe sepsis” (or today, in the era of Sepsis-3, simply “sepsis”).
An accurate understanding of epidemiologic trends in sepsis is important to guide new research and investment priorities as well as to provide credible assessments of quality improvement initiatives. This is particularly relevant today given the increasing regulatory focus on improving sepsis care and public reporting of sepsis bundle compliance and outcomes (17). In this review, we summarize the major epidemiologic studies of sepsis trends, potential biases in these analyses, and recent work that has utilized large electronic health record datasets to more objectively characterize sepsis trends using consistent clinical criteria.
Epidemiologic studies based on administrative data
Most of the studies examining population-level trends in sepsis incidence have used large administrative databases and examined in-hospital mortality as an outcome (Table 1). In 2001, Martin et al. described sepsis trends in the United States from 1979 through 2000 using administrative data from the National Hospital Discharge Survey, which included data from approximately 500 hospitals and 1% of all hospitalizations (1). This analysis, which utilized International Classification International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for septicemia, bacteremia, and disseminated fungal infections, demonstrated an increase from 164,000 sepsis cases in 1979 to nearly 660,000 in 2000 (an annual increase of 8.7%) with a concurrent decrease in-hospital mortality from 27.8% in 1979–1984 to 17.9% in 1995–2000. The ICD-9-CM codes were validated through a nested case-control analysis of 72 patients admitted to a large university hospital; medical record review yielded a positive predictive value of 89% and a negative predictive value of 80%. Notably, however, there was no analysis of whether the performance of those codes might have changed over the timeframe of the study.
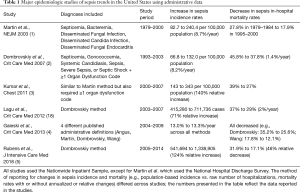
Full table
Dombrovskiy et al. subsequently examined sepsis trends in the U.S. from 1993 to 2003 using the Nationwide Inpatient Sample, which includes approximately 20% of nonfederal hospitals weighted to produce national estimates (2). The ICD-9-CM codes differed from the Martin study and required discharge diagnosis codes for (I) septicemia, or one of the new sepsis-specific codes introduced in 2002 (sepsis, severe sepsis, septic shock), and (II) at least one code indicating major organ dysfunction. This analysis demonstrated that age-adjusted incidence rates for sepsis hospitalizations doubled from 64.7 per 100,000 persons in 1993 to 134.6 in 2003, while mortality rates decreased from 45.8% to 37.8%. Kumar et al. later used the same data source to extend the estimates published by Martin et al. (using concurrent codes for infection and organ failure) and described an increase in sepsis hospitalizations from 143 per 100,000 persons in 2000 to 343 in 2007 (3). This study also documented an increase in the mean number of organ failures in sepsis hospitalizations from 1.6 to 1.9 and a decline in case fatality rates from 39% to 27%.
In 2013, Gaieski et al. examined sepsis trends from 2004–2009 by applying 4 different published administrative definitions to the Nationwide Inpatient Sample. This included the ICD-9-CM codes used by Martin et al., Dombrovskiy et al., Angus et al. (which used a broader set of infection codes in conjunction with organ dysfunction codes) (19), and Wang et al. (which combined similar codes as Angus et al. with measured temperature and hypotension in the emergency department) (20). The annual incidence varied by as much as 3.5-fold depending on the method used, but the average annual increase in incidence was approximately 13% across all methods. Mortality rates also varied substantially across methods but similar annual decreases were seen over time; for example, mortality rates using the Dombrovskiy codes fell from 35.2% to 25.6% and from 17.8% to 12.1% using the Wang method. This study also demonstrated that use of the new ICD-9-CM sepsis-specific codes more than doubled over the 6-year study period.
A more recent analysis by Rubens et al. demonstrated that these trends have continued in the U.S. from 2005–2014, with an increase in hospitalizations with Dombrovskiy codes from 541,649 to 1,338,905 during that time period (5). For comparison, the Dombrovskiy study identified 168,239 sepsis cases in 1993; thus, administrative data suggest a nearly 8-fold increase in sepsis incidence over a 20-year period, a finding that cannot be plausibly explained by a true increase in disease rates alone. Notably, investigators in other countries have reported similar trends using administrative data, including in several European and Asian countries (21-25).
Potential biases in sepsis trends derived from administrative data
While administrative data are readily available and convenient to analyze, they are susceptible to major biases that can yield misleading impressions about changes in sepsis rates and outcomes. These biases are discussed below and are summarized in Figure 1.
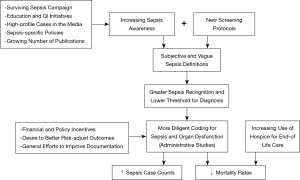
Increasing sepsis awareness, recognition, and coding
Increasing awareness of sepsis among providers is a major focus of the Surviving Sepsis Campaign and of many quality improvement initiatives that have proliferated in hospitals around the world (26). These initiatives typically include education and new screening protocols designed to increase sepsis recognition. It is likely that many of the patients that are newly identified as “sepsis” will be milder cases that would previously have only been labeled with their primary infection (e.g., pneumonia) (17,27). Public awareness campaigns have also contributed; for example, an analysis of web searches for sepsis reported by Google Trends demonstrated that interest in sepsis rose by more than 50% from 2012–2017, with recurrent cyclic increases that were correlated with World Sepsis Day and other media events (28).
Hospitals are also continually engaging in efforts to improve physician documentation and coding in order to better record patients’ clinical conditions, improve risk-adjusted mortality estimates for benchmarking, and optimize reimbursement (29-33). This is particularly relevant since sepsis is assigned a higher severity-of-illness rank, and thus reimbursement, compared to other infections. Underscoring the potential influence of policies and reimbursement on sepsis trends, Gohil et al. analyzed administrative data from California hospitals from 2000–2010 and found that increases in sepsis incidence were temporally correlated with new federal coding guidance and the introduction of medical severity diagnosis-related groups (MS-DRG) in 2007 (34). Under MS-DRG, sepsis was among the top 10 DRGs with increasing payments; furthermore, the introduction of the MS-DRG system led to national education efforts to improve physician documentation on the complexity and severity-of-illness of their patients (29).
Another single-center study examined nearly 100,000 hospitalizations from 2008–2012 in which systemic inflammatory syndrome criteria were met, and estimated changes in the probability of getting diagnosed, documented, and coded for sepsis during the study period after adjusting for demographics, comorbidities, frequency of blood culture draws, and intensive care unit admission (35). This analysis found that patients with similar characteristics and risk factors had a higher probability of being labeled with sepsis with each successive year, contributing to an apparent increase in sepsis incidence.
Lack of a gold standard test and subjectivity in diagnosing sepsis
The impact of increasing sepsis awareness and coding on reported trends is compounded by the lack of a “gold standard” diagnostic test for sepsis and the highly subjective nature of sepsis clinical criteria (36,37). Diagnosing sepsis (previously “severe sepsis”) requires clinicians to decide whether or not infection is present and whether organ dysfunction is present and attributable to infection versus other acute processes (such as dehydration, volume overload, medications, or other diseases). It is therefore unsurprising that experienced clinicians often disagree as to whether or not sepsis is present (38,39). This subjectivity grants both clinicians and hospitals broad discretion when assigning diagnoses and administrative codes for sepsis.
Decreasing threshold to diagnose and code for organ dysfunction
The threshold to diagnose and code for organ dysfunction also appears to be decreasing over time (40). This is relevant for epidemiologic studies since many administrative definitions of sepsis allow the disease to be defined by either explicit sepsis codes or the simultaneous presence of both infection and organ dysfunction codes; furthermore, a decreasing threshold to diagnose organ dysfunction can lead to a false impression that sepsis severity is increasing. We conducted a retrospective analysis using electronic health record data from patients hospitalized from 2005–2013 at two U.S. hospitals and found that the sensitivity of discharge codes for identifying hospitalizations with acute organ dysfunction (defined by consistent clinical criteria, such as vasopressors, mechanical ventilation, and unambiguous laboratory value thresholds) increased steadily over time, most strikingly for acute kidney failure and respiratory failure (40). There was also a simultaneous decrease in the positive predictive value for most types of organ dysfunction codes relative to strict clinical criteria, suggesting increasing use of organ dysfunction codes for milder forms of organ dysfunction over time. These findings were in-line with an analysis by Lagu et al. that applied the Dombrovskiy administrative definition to national data from 2003–2007 and found a paradoxical increase in the number of dysfunctional organ systems in patients with sepsis but decreasing in-hospital mortality rates and mean costs per case (18).
Increasing use of hospice for end-of-life care
The use of hospice services has increased substantially in the U.S. over the past several decades (41,42). This evolving societal preference for hospice care at the end of life also applies to patients with sepsis, particularly since many of the patients who die from sepsis have severe underlying comorbidities such as cancer (43). This trend can bias estimates of short-term mortality rates since most administrative studies have reported in-hospital mortality as the primary outcome for patients with sepsis. At least two studies have demonstrated that the trend of declining mortality rates for patients with sepsis is attenuated when using a combined outcome of in-hospital death or discharge to hospice as opposed to in-hospital death alone (44,45).
Sepsis epidemiologic studies based on clinical registries
Not every report of rising sepsis incidence and declining mortality is based on administrative data. One of the largest and most rigorous studies of sepsis epidemiology was conducted by Kaukonen et al. in Australia and New Zealand using a database of 1 million patients admitted to 171 ICUs from 2000 through 2012 (46). The study investigators determined whether each patient had sepsis, defined by 2 or more systemic inflammatory syndrome criteria and either (I) an APACHE III diagnosis of sepsis or septic shock, or (II) an APACHE III admission diagnosis of infection and at least 1 organ failure (defined in a consistent manner over the study period using the Sequential Organ Failure Assessment score) within 24 hours after ICU admission. The number of sepsis cases increased each year as a proportion of total ICU admissions from 7.2% in 2000 to 11.1%, while in-hospital mortality rates decreased from 35.0% in 2000 to 18.4% in 2012. Mortality improvements persisted after controlling for age, demographics, and severity-of-illness, and findings were consistent on a sensitivity analysis of the 63 ICUs that contributed data across all years.
Improvements in sepsis-associated mortality have also been reported in a large cohort of patients in the Surviving Sepsis Campaign database, which includes manually screened patients with suspected infection, 2 or more systemic inflammatory response syndrome criteria, and 1 or more organ dysfunction criteria (9,47). Similar improvements have been reported in quality improvement initiatives and after the implementation of mandatory sepsis protocols in New York State (9-13,48).
Potential biases with clinical registries
Clinical registries are likely more rigorous with sepsis assignments than administrative coding, especially if organ failure criteria are abstracted in a consistent manner. However, this surveillance method is still susceptible to suggesting misleading rises in sepsis rates and declines in mortality because of increasing sepsis awareness, recognition, and preferential admission of sepsis cases to ICUs over time. In the Kaukonen study, a diagnosis of sepsis on admission to the ICU was one of the inclusion criteria; given the subjectivity and imprecision of this diagnosis, the analysis is likely biased by a decreasing threshold to diagnosis sepsis over time (as with administrative studies). Indeed, while there was a substantial increase in the number of patients included in the study in each year (rising from 2,708 in the year 2000 to 12,512 in the year 2012), there was a parallel decrease in patients’ acuity of illness; for example, in the first year of the study, 30% of patients had APACHE III scores in the top quartile (i.e., >87) but by the end of the study, only 21% of patients had APACHE III scores in the top quartile.
In prospective registries like the Surviving Sepsis Campaign database or other quality improvement initiatives, ascertainment bias is particularly problematic because screening tools are designed to increase suspicion of infection and all patients with suspected infection may then counted, not just those in whom infection is confirmed or even probable. Underscoring this limitation, one healthcare system reported a reduction in sepsis mortality by more than 50% after implementing a quality improvement initiative, but twice as many sepsis cases were included in their denominator in the post-initiative period (11).
ICU-based analyses like the Kaukonen study also ignore the many patients with sepsis who are never admitted to the ICU (49), and could further be biased by changes in ICU admission thresholds over time. Lastly, in the Kaukonen study, only in-hospital mortality was examined, with no information reported on trends in discharge to hospice or mortality shortly after hospital discharge.
Analyses of mortality trends in sepsis randomized controlled trials
Stevenson et al. examined trends in mortality in the usual care arm of 36 multicenter randomized trials of sepsis from 1991 to 2009, reasoning that this might offer more objective insight into sepsis mortality trends since these patients are more rigorously selected compared to studies based on administrative data (50). They found that mortality rates declined by 3% annually, mirroring declines in mortality reported by administrative data (50). Importantly, though, sepsis mortality rates decreased from 1991 to 2000 but then increased from 26.6% in 2001–2005 to 29.2% in 2006–2009, calling into question their conclusion that sepsis mortality rates are indeed steadily decreasing.
Luhr et al. similarly analyzed the usual care arms of 44 randomized trials of sepsis published between 2002 and 2016, but came to a different conclusion (51). They found a much milder mortality decline at 0.42% annually (vs. 3% with the Stevenson study), yielding a total decline of 9.2% over the study period. However, after adjusting for severity-of-illness (including APACHE II, SAPS II, and SOFA scores when available), the observed trends were non-significant. They concluded that the decrease in sepsis mortality in these trials was likely in large part due to the inclusion of less severely ill patients over time. Although this study makes no inferences about trends in sepsis incidence, the finding that a gradually declining severity-of-illness may partially or fully explain declining sepsis mortality trends is consistent with the hypothesis that the threshold to label a patient as septic is decreasing and thus sepsis denominators are increasing.
Objective sepsis surveillance using clinical data from electronic health records
Given the limitations of administrative data and clinical registries, alternate surveillance methods are needed that are more objective and can be easily applied to large populations in a sustained fashion. The increasing ubiquity of electronic health record (EHR) systems around the nation allows for the possibility of conducting widespread surveillance for sepsis using consistent clinical criteria for infection and concurrent organ dysfunction (52). Below, we summarize several studies that have utilized this framework to better understand and track trends in sepsis incidence and outcomes.
Trends in sepsis coding, incidence, and mortality using EHR data from academic hospitals
We used EHR data from two U.S. academic hospitals to examine trends in hospitalizations with positive blood cultures and concurrent vasopressors or lactic acidosis on the rationale that these are unambiguous cases of septic shock (53). We demonstrated that while the incidence of hospitalizations with sepsis diagnosis codes increased dramatically from 2003–2012, there was no concurrent increase in number of patients with bacteremic shock. We also found that the sensitivity of sepsis codes for identifying hospitalizations with bacteremic shock increased over time. Furthermore, coding for “septicemia”—a vague term that has traditionally implied bacteremia with clinical signs of sepsis (54,55)—was increasingly applied to patients without documented positive blood cultures. These findings provide evidence that sepsis diagnosis codes are becoming increasingly sensitive over time and are being extended to broader populations.
Using the same dataset, we developed a more comprehensive surveillance definition for sepsis based on concurrent clinical markers of presumed infection (blood culture orders and antibiotic administrations) and organ dysfunction (vasopressors, mechanical ventilation, and changes in baseline laboratory values) and demonstrated that this definition has superior sensitivity and comparable specificity versus administrative codes relative to physician medical record reviews (56). Importantly, the surveillance definition had stable performance during an earlier time period (2003–2009) versus a later period (2012), whereas the sensitivity of administrative definitions increased over time. When applying these criteria to all EHR data from the two U.S. hospitals, sepsis incidence and mortality rates changed much less dramatically from 2003–2012 than suggested by administrative data.
Septic shock trends using EHR data from 27 academic hospitals
We then tested a surveillance definition for septic shock based on concurrent blood culture sampling, antibiotics, and ≥2 days of vasopressors. This definition had higher sensitivity than septic shock ICD-9-CM codes and comparable positive predictive value (44). When applied to clinical data from 27 U.S. academic hospitals between 2005 and 2014, septic shock incidence by clinical criteria rose an average of 4.9% per year (95% CI: 4.0–5.9%) while mortality declined by 0.6% per year (95% CI: 0.4–0.8%). In contrast, septic shock incidence based on ICD-9-CM codes increased by 19.8% per year (95% CI: 16.6–20.9%) and mortality declined by 1.2% per year (0.9–1.6%). The findings were robust to sensitivity analyses that required ≥1 day of vasopressors (rather than 2 days) and varied the required number of days of antibiotics or length of the surveillance window.
Sepsis trends using EHR data from a nationally representative dataset
In the largest study to date assessing sepsis epidemiology using EHR data, we estimated trends from a nationally representative set of academic, community, and federal hospitals (45). In doing so, we sought to adapt our prior work to maintain parity with the newly released Sepsis-3 consensus clinical definition (57), but focused on clinical indicators of infection and organ dysfunction that are objective, routinely measured or used to treat sepsis, easily ascertainable from diverse EHRs, and suitable for consistent and uniform application across different hospitals. Specifically, we defined presumed serious infection as ≥1 blood culture draw and concurrent administration of ≥4 consecutive days of antimicrobials (fewer if patients died or were transferred to hospice or another acute care hospital), and identified concurrent organ dysfunction through a modified version of the SOFA score that dichotomizes organ dysfunction as present or absent for each organ system (Table 2). A minimum of four antibiotic days was chosen as a threshold for presumed infection to minimize false positives from patients who have empiric treatment stopped when an initially suspected infection is not confirmed. The organ dysfunction thresholds generally corresponded to a SOFA score increase of ≥2 points but the criteria simplified or eliminated SOFA components that may be inconsistently measured, documented, and stored in EHRs, such as vital signs, vasopressor doses, urine output, arterial blood gases, fraction of inspired oxygen, and Glasgow Coma Scale.

Full table
Validation using medical record reviews indicated that the EHR-based definition had 70% sensitivity and 70% positive predictive value for identifying hospitalizations meeting Sepsis-3 criteria (45). Positive predictive value was 88% when sepsis was defined as organ dysfunction concurrent with clinically suspected (rather than confirmed) infection. In contrast, “explicit” diagnosis codes for severe sepsis or septic shock were less sensitive (33%) with similar positive predictive value (76%), while “implicit” codes for infection and organ dysfunction had comparable sensitivity (66%) but lower positive predictive value (31%). Medical record reviews suggested that the Sepsis-3 cases missed by EHR clinical criteria had mild organ dysfunction (such as hypoxemia without need for mechanical ventilation) and low risk of mortality.
We then applied the clinical surveillance definition to EHR data from 409 hospitals. We found that sepsis incidence from 2009–2014 was stable (+0.6% relative change/year, 95% CI: −2.3%, +3.5%, P=0.67) whereas incidence per explicit sepsis codes increased (+10.3%/year, 95% CI: 7.2%, 13.3%, P<0.001) (Figure 2). In-hospital mortality using EHR clinical criteria declined (−3.3%/year, 95% CI: −5.6%, −1.0%, P=0.004) but there was no significant change in the combined outcome of death or discharge to hospice (−1.3%/year, 95% CI: −3.2%, +0.6%, P=0.19). In contrast, mortality using explicit sepsis codes declined significantly (−7.0%/year, 95% CI: −8.8%, −5.2%, P<0.001) as did death or discharge to hospice (−4.5%/year, 95% CI: −6.1%, −2.8%, P<0.001). When sepsis hospitalizations were identified using implicit codes, the absolute incidence was higher and mortality lower compared to explicit sepsis codes, but trends were similar. Among sepsis patients identified using EHR clinical criteria, there was an increase over time in the proportion assigned sepsis codes (from 24.9% in 2009 to 30.5% in 2014), indicating growing sepsis awareness, documentation, and coding.
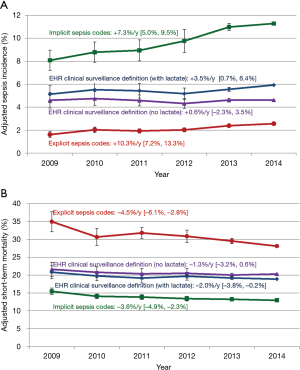
In the primary analysis of trends, an elevated lactate level was not included as one of the qualifying organ dysfunction criteria, due to the risk that increasing rates of lactate testing over time might cause an ascertainment bias (58). This was borne out in a sensitivity analysis that included elevated lactate in the surveillance definition, which demonstrated mild increases in sepsis incidence and more substantial decreases in mortality or discharge to hospice (though still less than observed with administrative data).
The surveillance definition utilized in this study has since been incorporated into CDC’s “Adult Sepsis Event” toolkit, which is aimed at helping hospitals better track their sepsis rates and outcomes using clinical data rather than administrative data (59).
Limitations of EHR-based surveillance
Although EHR-based surveillance is more objective than administrative data or even perhaps prospective screening methods (since it does not rely on clinicians making the diagnosis of sepsis), it is still susceptible to variation in practice patterns between clinicians and changes over time because it incorporates clinical judgments such as the decision to draw a blood culture, check lactates, administer antibiotics, continue antibiotics, intubate, and/or start vasopressors. In particular, including a criterion for elevated lactate can cause ascertainment bias if lactate testing rates increase over time. CDC’s Adult Sepsis Event definition is also subject to misclassification since some patients who receive ≥4 days of antibiotics may not have truly been infected, and some patients may have organ dysfunction for reasons unrelated to infection. Importantly, though, the Adult Sepsis Event temporally associates presumed infection with organ dysfunction in a consistent manner. Further work is also needed to confirm the generalizability of this method to diverse hospitals around the world and to assess the consistency of its association with Sepsis-3 criteria.
Conclusions
Numerous studies have suggested that sepsis incidence is increasing over time and mortality rates are declining. These estimates are biased, however, by increasing clinical awareness of sepsis, more screening, decreasing diagnostic thresholds, and more diligent coding practices. The net effect of these many sources of bias is misleading impressions of rising sepsis incidence and decreasing sepsis mortality rates. Recent work identifying sepsis using direct clinical indicators of infection and organ dysfunction instead of administrative codes or registries suggest that sepsis incidence and mortality are in fact relatively stable over the past decade. This method of sepsis surveillance can be applied to routine data present in EHR systems and thus provides a credible and efficient means of continuous monitoring of sepsis trends and outcomes across large numbers of hospitals. Objective sepsis surveillance using EHR data has the potential to help clinicians, quality officers, policy makers, and public health officials to better monitor the impact of quality improvement and policy initiatives, identify additional risk factors and targets for prevention, and guide new programs and research investments.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003;348:1546-54. [Crossref] [PubMed]
- Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med 2007;35:1244-50. [Crossref] [PubMed]
- Kumar G, Kumar N, Taneja A, et al. Nationwide trends of severe sepsis in the 21st century (2000-2007). Chest 2011;140:1223-31. [Crossref] [PubMed]
- Gaieski DF, Edwards JM, Kallan MJ, et al. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 2013;41:1167-74. [Crossref] [PubMed]
- Rubens M, Saxena A, Ramamoorthy V, et al. Increasing Sepsis Rates in the United States: Results From National Inpatient Sample, 2005 to 2014. J Intensive Care Med 2018.885066618794136. [Epub ahead of print]. [PubMed]
- Danai PA, Moss M, Mannino DM, et al. The epidemiology of sepsis in patients with malignancy. Chest 2006;129:1432-40. [Crossref] [PubMed]
- Girard TD, Opal SM, Ely EW. Insights into severe sepsis in older patients: from epidemiology to evidence-based management. Clin Infect Dis 2005;40:719-27. [Crossref] [PubMed]
- Esper AM, Martin GS. Extending international sepsis epidemiology: the impact of organ dysfunction. Crit Care 2009;13:120. [Crossref] [PubMed]
- Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010;38:367-74. [Crossref] [PubMed]
- Cannon CM, Holthaus CV, Zubrow MT, et al. The GENESIS project (GENeralized Early Sepsis Intervention Strategies): a multicenter quality improvement collaborative. J Intensive Care Med 2013;28:355-68. [Crossref] [PubMed]
- Miller RR 3rd, Dong L, Nelson NC, et al. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med 2013;188:77-82. [Crossref] [PubMed]
- Whippy A, Skeath M, Crawford B, et al. Kaiser Permanente's performance improvement system, part 3: multisite improvements in care for patients with sepsis. Jt Comm J Qual Patient Saf 2011;37:483-93. [Crossref] [PubMed]
- Micek ST, Roubinian N, Heuring T, et al. Before-after study of a standardized hospital order set for the management of septic shock. Crit Care Med 2006;34:2707-13. [Crossref] [PubMed]
- Klompas M, Rhee C. We Need Better Tools for Sepsis Surveillance. Crit Care Med 2016;44:1441-2. [Crossref] [PubMed]
- Klompas M, Rhee C. Sepsis and the theory of relativity: measuring a moving target with a moving measuring stick. Crit Care 2016;20:396. [Crossref] [PubMed]
- Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med 1985;312:1604-8. [Crossref] [PubMed]
- Rhee C, Gohil S, Klompas M. Regulatory mandates for sepsis care--reasons for caution. N Engl J Med 2014;370:1673-6. [Crossref] [PubMed]
- Lagu T, Rothberg MB, Shieh MS, et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med 2012;40:754-61. [Crossref] [PubMed]
- Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303-10. [Crossref] [PubMed]
- Wang HE, Shapiro NI, Angus DC, et al. National estimates of severe sepsis in United States emergency departments. Crit Care Med 2007;35:1928-36. [Crossref] [PubMed]
- Fleischmann-Struzek C, Mikolajetz A, Schwarzkopf D, et al. Challenges in assessing the burden of sepsis and understanding the inequalities of sepsis outcomes between National Health Systems: secular trends in sepsis and infection incidence and mortality in Germany. Intensive Care Med 2018;44:1826-35. [Crossref] [PubMed]
- Lee CC, Yo CH, Lee MG, et al. Adult sepsis - A nationwide study of trends and outcomes in a population of 23 million people. J Infect 2017;75:409-19. [Crossref] [PubMed]
- Oh SY, Cho S, Kim GH, et al. Incidence and Outcomes of Sepsis in Korea: A Nationwide Cohort Study From 2007 to 2016. Crit Care Med 2019;47:e993-8. [Crossref] [PubMed]
- Wilhelms SB, Huss FR, Granath G, et al. Assessment of incidence of severe sepsis in Sweden using different ways of abstracting International Classification of Diseases codes: difficulties with methods and interpretation of results. Crit Care Med 2010;38:1442-9. [Crossref] [PubMed]
- Bouza C, Lopez-Cuadrado T, Saz-Parkinson Z, et al. Epidemiology and recent trends of severe sepsis in Spain: a nationwide population-based analysis (2006-2011). BMC Infect Dis 2014;14:3863. [Crossref] [PubMed]
- Slade E, Tamber PS, Vincent JL. The Surviving Sepsis Campaign: raising awareness to reduce mortality. Crit Care 2003;7:1-2. [Crossref] [PubMed]
- Lindenauer PK, Lagu T, Shieh MS, et al. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003-2009. JAMA 2012;307:1405-13. [Crossref] [PubMed]
- Jabaley CS, Blum JM, Groff RF, et al. Global trends in the awareness of sepsis: insights from search engine data between 2012 and 2017. Crit Care 2018;22:7. [Crossref] [PubMed]
- Rosenstein AH, O'Daniel M, White S, et al. Medicare's value-based payment initiatives: impact on and implications for improving physician documentation and coding. Am J Med Qual 2009;24:250-8. [Crossref] [PubMed]
- Hicks TA, Gentleman CA. Improving physician documentation through a clinical documentation management program. Nurs Adm Q 2003;27:285-9. [Crossref] [PubMed]
- Grogan EL, Speroff T, Deppen SA, et al. Improving documentation of patient acuity level using a progress note template. J Am Coll Surg 2004;199:468-75. [Crossref] [PubMed]
- Spellberg B, Harrington D, Black S, et al. Capturing the diagnosis: an internal medicine education program to improve documentation. Am J Med 2013;126:739-43.e1. [Crossref] [PubMed]
- Richter E, Shelton A, Yu Y. Best practices for improving revenue capture through documentation. Healthc Financ Manage 2007;61:44-7. [PubMed]
- Gohil SK, Cao C, Phelan M, et al. Impact of Policies on the Rise in Sepsis Incidence, 2000-2010. Clin Infect Dis 2016;62:695-703. [Crossref] [PubMed]
- Jafarzadeh SR, Thomas BS, Marschall J, et al. Quantifying the improvement in sepsis diagnosis, documentation, and coding: the marginal causal effect of year of hospitalization on sepsis diagnosis. Ann Epidemiol 2016;26:66-70. [Crossref] [PubMed]
- Angus DC, Seymour CW, Coopersmith CM, et al. A Framework for the Development and Interpretation of Different Sepsis Definitions and Clinical Criteria. Crit Care Med 2016;44:e113-21. [Crossref] [PubMed]
- Seymour CW, Coopersmith CM, Deutschman CS, et al. Application of a Framework to Assess the Usefulness of Alternative Sepsis Criteria. Crit Care Med 2016;44:e122-30. [Crossref] [PubMed]
- Rhee C, Kadri SS, Danner RL, et al. Diagnosing sepsis is subjective and highly variable: a survey of intensivists using case vignettes. Crit Care 2016;20:89. [Crossref] [PubMed]
- Lopansri BK, Miller Iii RR, Burke JP, et al. Physician agreement on the diagnosis of sepsis in the intensive care unit: estimation of concordance and analysis of underlying factors in a multicenter cohort. J Intensive Care 2019;7:13. [Crossref] [PubMed]
- Rhee C, Murphy MV, Li L, et al. Improving documentation and coding for acute organ dysfunction biases estimates of changing sepsis severity and burden: a retrospective study. Crit Care 2015;19:338. [Crossref] [PubMed]
- Han B, Remsburg RE, McAuley WJ, et al. National trends in adult hospice use: 1991-1992 to 1999-2000. Health Aff (Millwood) 2006;25:792-9. [Crossref] [PubMed]
- Frahm KA, Barnett SD, Brown LM. Trends in hospice utilization across age among the veteran population. Am J Hosp Palliat Care 2011;28:424-8. [Crossref] [PubMed]
- Rhee C, Jones TM, Hamad Y, et al. Prevalence, Underlying Causes, and Preventability of Sepsis-Associated Mortality in US Acute Care Hospitals. JAMA Netw Open 2019;2:e187571. [Crossref] [PubMed]
- Kadri SS, Rhee C, Strich JR, et al. Estimating Ten-Year Trends in Septic Shock Incidence and Mortality in United States Academic Medical Centers Using Clinical Data. Chest 2017;151:278-85. [Crossref] [PubMed]
- Rhee C, Dantes R, Epstein L, et al. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009-2014. JAMA 2017;318:1241-9. [Crossref] [PubMed]
- Kaukonen KM, Bailey M, Suzuki S, et al. Mortality Related to Severe Sepsis and Septic Shock Among Critically Ill Patients in Australia and New Zealand, 2000-2012. JAMA 2014;311:1308-16. [Crossref] [PubMed]
- Levy MM, Rhodes A, Phillips GS, et al. Surviving sepsis campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med 2015;43:3-12. [Crossref] [PubMed]
- Levy MM, Gesten FC, Phillips GS, et al. Mortality Changes Associated with Mandated Public Reporting for Sepsis. The Results of the New York State Initiative. Am J Respir Crit Care Med 2018;198:1406-12. [Crossref] [PubMed]
- Rohde JM, Odden AJ, Bonham C, et al. The epidemiology of acute organ system dysfunction from severe sepsis outside of the intensive care unit. J Hosp Med 2013;8:243-7. [Crossref] [PubMed]
- Stevenson EK, Rubenstein AR, Radin GT, et al. Two Decades of Mortality Trends Among Patients With Severe Sepsis: A Comparative Meta-Analysis. Crit Care Med 2014;42:625-31. [Crossref] [PubMed]
- Luhr R, Cao Y, Soderquist B, et al. Trends in sepsis mortality over time in randomised sepsis trials: a systematic literature review and meta-analysis of mortality in the control arm, 2002-2016. Crit Care 2019;23:241. [Crossref] [PubMed]
- Rhee C, Dantes RB, Epstein L, et al. Using objective clinical data to track progress on preventing and treating sepsis: CDC's new 'Adult Sepsis Event' surveillance strategy. BMJ Qual Saf 2019;28:305-9. [Crossref] [PubMed]
- Rhee C, Murphy MV, Li L, et al. Comparison of Trends in Sepsis Incidence and Coding Using Administrative Claims Versus Objective Clinical Data. Clin Infect Dis 2015;60:88-95. [Crossref] [PubMed]
- Bone RC. Let's agree on terminology: definitions of sepsis. Crit Care Med 1991;19:973-6. [Crossref] [PubMed]
- Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644-55. [Crossref] [PubMed]
- Rhee C, Kadri S, Huang SS, et al. Objective Sepsis Surveillance Using Electronic Clinical Data. Infect Control Hosp Epidemiol 2016;37:163-71. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Rhee C, Murphy MV, Li L, et al. Lactate Testing in Suspected Sepsis: Trends and Predictors of Failure to Measure Levels. Crit Care Med 2015;43:1669-76. [Crossref] [PubMed]
- Centers for Disease Control and Prevention: Hospital Toolkit for Adult Sepsis Surveillance. Available online: . Accessed December 1st, 2019.https://www.cdc.gov/sepsis/pdfs/Sepsis-Surveillance-Toolkit-Mar-2018_508.pdf


