Report on 153 sequential three-incision robotic-assisted pulmonary resections by a single surgeon: technical details and learning curve
Introduction
Robot-assisted surgery first came into use in the mid-1990s. One of its aims was to overcome the limitations of thoracoscopic surgery, which included a narrow field of view, restricted freedom of movement, and poor ergonomics. First described for human use by Cadiere et al. in 1999, robot-assisted surgery (1), with its three-dimensional (3D) visualization, improved camera quality, wristed instruments, and ergonomic ease, has become widespread in the treatment of a number of conditions. The first robot-assisted thoracic surgery was described by Melfi et al. in 2002 (2), and several studies have shown that robotic lobectomy for lung cancer affords both a radicality and safety comparable to those of video-assisted thoracic surgery (VATS) and open surgery (3). Given its useful features and positive outcomes, robotic-assisted pulmonary resection has been increasingly practiced worldwide.
There are several different techniques within the robotic pulmonary resection method. In 2006, Park et al. developed an approach for robotic-assisted lobectomy with two 1- to 1.5-cm access incisions and a 4-cm utility incision, which conformed to the standard VATS lobectomy technique (4). In 2010, Veronesi et al. introduced a four-arm robotic-assisted lobectomy technique using three-port incisions and a 3- to 4-cm utility incision (5). In 2009, Gharagozloo et al. reported a hybrid technique which included a robotic dissection phase and VATS lobectomy phase (6). Four incisions were used, including three 2- to 3-cm incisions and an additional 1- to the 2-cm incision. The process includes robotic vascular, hilar, and mediastinal dissection, followed by VATS lobectomy. In 2010, Ninan and Dylewski reported on the effectiveness of complete portal robotic lobectomy (CPRL) using 3 arms (7). In 2014, Cerfolio introduced a CPRL 4-arm approach (8) in which pneumothorax was induced by CO2 insufflation, and a utility incision to remove the surgical specimen was made at the end of the procedure.
The placement of the trocars and the utility incision in the right position is critical for thoracic surgeons to obtain the best performance of the operation and avoid arm impingement and interference. Fewer incisions may help to reduce postoperative pain and improve quality of life while using fewer arms reduces the cost of operation. In this report, we introduce our three-incision robotic technique for lobectomy, segmentectomy, and lymph node removal, and evaluate the feasibility and safety in the treatment of non-small cell lung cancer.
Methods
Patient positioning and port placement
The procedure is performed under general anesthesia and single-lung ventilation achieved by a double-lumen endotracheal tube or a bronchial occluder. The patient is positioned in a lateral-decubitus-with-jackknife position to achieve maximum separation of the intercostal spaces (ICSs). The da Vinci Si robot is positioned at the head of the patient (Figure 1).
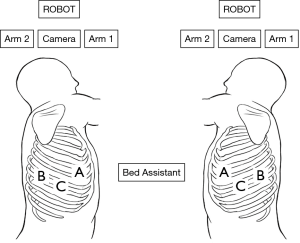
Using the three-incision technique, two-port incisions, and a 3-cm utility incision are made at the positions indicated in Figure 1. The 12-mm camera port is placed at the level of the mid-posterior axillary line in the 7th or 8th ICS. After exploring the pleural cavity with the 30-degree stereoscopic robotic camera, the second port (8 mm) is placed in the 7th or 8th ICS of the scapular line under view guidance. The utility incision, which is used for suctioning, retracting, and taking the specimens out, is made at the 5th or 6th ICS of the anterior axillary line and protected by the XINPIFORM® soft tissue retractor (Victor Medical, Changzhou, China). The anterior trocar is positioned at the inner margin of the utility incision, and the posterior trocar is positioned at the second port (Figure 2). The right and left trocars are 8–10 cm away from the camera.
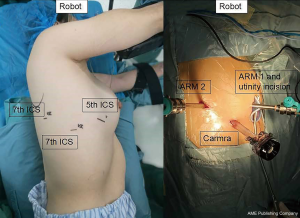
Three robotic arms are then docked to their respective trocars (Figure 3). A 30-degree stereoscopic robotic camera is placed in the middle arm, a permanent cautery hook is placed in the right robotic arm (surgeon right hand, arm 1), and a fenestrated bipolar forceps is placed in the left robotic arm (surgeon left hand, arm 2). The permanent cautery hook is used for precise dissection and isolation of the pulmonary vascular structures, and the fenestrated bipolar forceps is used to retract the lobe and for hemostasis if necessary. The bed assistant uses sucker and ring forceps through the utility incision to help to improve the exposure of the operative field.
Lesions without a preoperative diagnosis are resected and subjected to intraoperative frozen section examination, while malignancy is followed by systematic lymph node dissection. Small or deep undiagnosed lesions can be localized by multiple methods, such as methylene blue injection, wire localization, or by injecting medical glue near the nodule under computed tomography (CT) guidance.
Dissection and division of hilar structures
The surgical steps were similar to those that are used in VATS with posterior-to-anterior hilum isolation. Usually, we first open the posterior pleura from the superior edge of the lower lobe vein to the inferior edge of the azygos vein, along the course of the vagus nerve. Lymph node station 7 (subcarinal) and station 11 (interlobar) are removed. Then, the pulmonary parenchyma is meticulously divided, and the ascending posterior segmental artery to the upper lobe and the superior segmental artery to the lower lobe are identified. This maneuver allows for the clear identification of the posterior parenchymal bridge. A tissue stapler passed through the utility incision is used to divide the posterior parenchymal bridge. This also helps to expose the common descending branch of the pulmonary artery. The arteries to the designated lobe are isolated and individually divided with a vascular stapler or Hem-O-Lok® clips (WECK, Teleflex Medical, NY, USA).
For a right upper lobectomy, dividing the right upper lobe bronchus facilitates the isolation of the truncus anterior branch of the pulmonary artery. The venous structures are typically divided last to avoid engorgement of the corresponding lobe. When performing a left upper lobectomy, the division of the left superior pulmonary vein will facilitate exposure of the bronchus and the apical and anterior arterial branches.
Mediastinal lymph node dissection
Radical lymph node dissection can be performed with even better visibility than in open surgery. For the removal of lymph node station 7 (subcarinal), the bed assistant uses sucker and ring forceps to retract the lung toward the anterior mediastinum. The fenestrated bipolar forceps is used to grab the mediastinal pleura posteriorly to expose the posterior mediastinum. Lymph nodes are removed en bloc using a permanent cautery hook.
Dissection of lymph node station 2 (upper paratracheal) and 4 (lower paratracheal) are performed on the right side, usually at the end of surgery. The bed assistant retracts the azygos vein and the superior vena cava to expose the target area. Paratracheal lymph nodes are then removed en bloc.
Ethics statement
All patient data were retrieved from hospital medical record system, and the study outcomes will not affect the future management of the patients. The study was approved by the ethics committee of our institution (2019S001), which waived the requirement for informed consent.
Results
Between December 2016 and December 2018, 153 consecutive patients underwent three incisions, robotic-assisted pulmonary resection in the Thoracic Surgery Department of the Second Xiangya Hospital of Central South University. All surgeries were performed by Dr. Yu. The patient characteristics are listed below (Table 1).
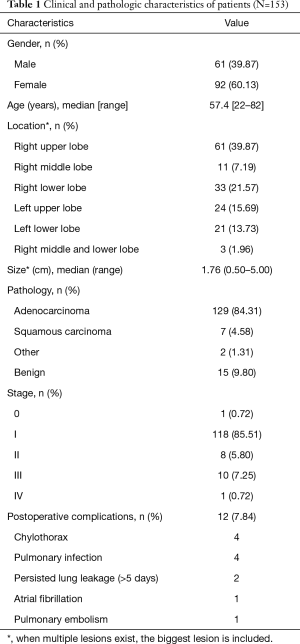
Full table
The procedures included 39 segmentectomies and 114 lobectomies. There were 138 malignancies and 15 benign lesions. Pathologic cell type of the malignancy was adenocarcinoma in 129 cases, squamous cell carcinoma in 7, pulmonary blastoma in 1, and metastatic epithelioid trophoblastic tumor in 1. There were 18 cases of multiple lung cancers among adenocarcinoma patients. The 15 benign lesions included 1 case of aspergillus, 1 case of bronchiectasis, 2 cased of tuberculosis, 2 cases of pulmonary sequestration, 2 cases of inflammatory myofibroblastic tumor, and 7 cases of chronic inflammation.
Clinical and pathologic staging was performed using the 8th edition of the TNM classification. One patient was found to have intrathoracic metastasis during surgery, and thus classified as stage IV. One patient with lung adenocarcinoma had a complete pathological remission after neoadjuvant treatment, and her stage was 0.
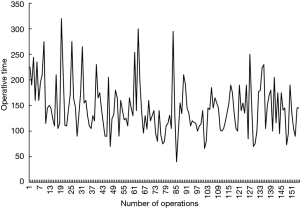
There was no emergent conversion to thoracotomy. None of these patients required blood transfusion intraoperatively or postoperatively. Operative time was measured from the start of incision to the end of skin closure, and thus included the time spent waiting for frozen section analysis. The median operative time was 146.84 minutes (range, 40–320 minutes) and as experience increased, the operative time decreased slowly (Figure 4). After 20 operations, the operation time entered the plateau period. The median estimated blood loss was 62.70 mL (range, 5–200 mL). The mean postoperative number of days before chest tubes were removed was 3.91 (range, 2–18), and the mean number of postoperative days before patients were discharged was 5.34 (range, 2–20). The median number of lymph node stations dissected was 5 (range, 1–9). An elderly woman with adenocarcinoma only had her hilar lymph nodes resected due to her age (80 years old). The mean number of nodes resected was 12 (range, 1–35).
Postoperative complications were observed in 12 patients (7.84%), and are noted in Table 1. The most common complication was chylothorax and pulmonary infection. All complications improved after conservative treatment. There were no in-hospital deaths, and the 30-day mortality rate was 0%.
Discussion
In the last few years, the robotic-assisted technique has been wildly adopted by thoracic surgeons for lung resection. The percentage of robotic lobectomies performed in non-academic US hospitals has increased from 1% in 2009 to 13% in 2013 (9). Each year, over 6,000 are now performed in the US, and 8,600 are performed worldwide (10,11).
Many retrospective studies have demonstrated that robotic-assisted pulmonary resection is feasible and safe, and initial long-term results indicate that the oncological outcome is comparable to that reported for open and VATS approaches (12-14). In 2016, Agzarian et al. conducted a comparative meta-analysis of robotic pulmonary resection and other modalities. Their results show a mean longer operative time of 61.69 minutes and 64.28 minutes for robotic-assisted thoracic surgery (RATS) vs. thoracotomy and VATS, respectively. There were no significant differences in conversion rates, prolonged air leak (PAL) rates, blood loss. and length of stay between RATS and VATS (3). In 2017, Oh et al. analyzed the Premier Healthcare Database to compare perioperative clinical outcomes from elective robotic-assisted lobectomy, video-assisted thoracoscopic lobectomy, and open lobectomy, with propensity score matching (1:1) for the patient and hospital characteristics of each group. Compared with video-assisted thoracoscopic lobectomy and open lobectomy, robotic-assisted lobectomy was associated with shorter length of stay and lower complication rates. It was also was associated with a lower conversion rate to open lobectomy compared with video-assisted thoracoscopic lobectomy (15).
Different approaches have been described for robotic pulmonary resection. However, a standard technique is still missing (Table 2). In 2017, the American Association of Thoracic Surgeons Writing Committee proposed a definition and nomenclature for robotic thoracic surgery. A robotic portal (RP) operation is defined as an operation that uses ports only, and the port incision(s) is/are not generally enlarged at any time during the operation to be a size larger than the trocars in them, except for when the specimen is removed. A robotic-assisted procedure is defined as an operation that includes a utility incision (31). According to this definition, robotic lobectomy can be divided into the robotic-assisted approach and the totally port-based approach. In the robotic-assisted approach, a utility incision, usually 3–4 cm, is created to help in the retraction, suction, and dissection by the table surgeon. This access port is also used for palpation of the nodule in case of wedge resection and to extract the large specimen. According to the number of arms, robotic-assisted lobectomy is divided into the 3-arm technique and the 4-arm technique. In the totally port-based approach, CO2 insufflation is used to facilitate lung collapse and drive the diaphragm inferiorly (8). This approach always needs four arms but does not need an experienced table surgeon. The palpation of the nodule is impossible in this procedure. This approach has higher initial capital costs and involves more specialized equipment than the robotic-assisted approach (27,32).
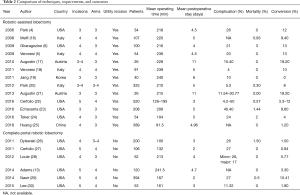
Full table
Our three-incision approach for robotic-assisted pulmonary resection differs from other 3-arm techniques in the choice of ICS for the port incisions and utility incisions. For example, Toker et al. placed the camera port on the 8th midaxillary ICS in the middle, the second port at the 8th or 9th ICS close to the paravertebral sulcus, and the anterior port on the 6th or 7th ICS. For the upper lobectomies and segmentectomies of the upper lobes, the access port is opened at the posterior ICS in the 10th or 11th ICS. For lower lobectomies and lower lobe segmentectomies, the anterior port is opened as the access port, and the table surgeon can share this access with the arm of the robot (32). This technique is very similar to ours, but there are subtle differences in the location of the ports (camera port on the 7th midaxillary ICS, anterior port on the 5th ICS, and the posterior ICS in the 7th ICS), while we only need three incisions for upper lung surgery.
There are three major reasons why we have adopted this three-incision technique. First, this approach is ideal for novices experienced in VATS surgery. The incisions are similar to conventional thoracoscopy, which facilitates the surgeon from becoming familiar with the structure and procedure. In the event of malfunction of the robot or the unavailability of timely system component repair, the remainder of the procedure can be performed with conventional thoracoscopy without the need for additional incisions. Second, this technique can improve the precision and comfort of the operation without increasing the trauma and incision, which is conducive to the postoperative recovery of patients. Meanwhile, reduced use of the robotic arms also helps to shorten operating time and save medical costs compared to a 4-arm technique.
Despite these advantages, a number of shortcomings do exist. First, compared to the 4-arm technique, using one armless can make the exposure slightly more difficult. It requires a highly trained assistant at the table and good teamwork between the console surgeon and table surgeon. Second, introducing staplers and instruments through the same port as the robotic arm hampers their mobility. The anterior arm needs to be de-docked under some circumstances, such as when dissecting the first branch of the pulmonary artery.
The technique we described here is used for the da Vinci Si systems. The emergence of new robot devices will address the existing technical problems (33), including the lack of force feedback and limited vision, which can also impact current incision selection. We expect that technologic advancement will further simplify the robotic procedure.
Acknowledgments
Funding: This study was supported by a grant from the Key Research and Development Program of Hunan Province (No. 2019SK2253).
Footnote
Conflicts of Interest: CC serves as the unpaid section editor of Journal of Thoracic Disease. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All patient data were retrieved from hospital medical record system, and the study outcomes will not affect the future management of the patients. The study was approved by the Ethics committee of our institution (No. 2019S001), which waived the requirement for informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cadiere GB, Himpens J, Vertruyen M, et al. The world's first obesity surgery performed by a surgeon at a distance. Obes Surg 1999;9:206-9. [Crossref] [PubMed]
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Agzarian J, Fahim C, Shargall Y, et al. The Use of Robotic-Assisted Thoracic Surgery for Lung Resection: A Comprehensive Systematic Review. Semin Thorac Cardiovasc Surg 2016;28:182-92. [Crossref] [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Gharagozloo F, Margolis M, Tempesta B, et al. Robot-assisted lobectomy for early-stage lung cancer: report of 100 consecutive cases. Ann Thorac Surg 2009;88:380-4. [Crossref] [PubMed]
- Ninan M, Dylewski MR. Total port-access robot-assisted pulmonary lobectomy without utility thoracotomy. Eur J Cardiothorac Surg 2010;38:231-2. [Crossref] [PubMed]
- Cerfolio RJ. Total port approach for robotic lobectomy. Thorac Surg Clin 2014;24:151-6. v. [Crossref] [PubMed]
- Linsky P, Wei B. Robotic lobectomy. J Vis Surg 2017;3:132. [Crossref] [PubMed]
- Suda T. Transition from video-assisted thoracic surgery to robotic pulmonary surgery. J Vis Surg 2017;3:55. [Crossref] [PubMed]
- Morgan JA, Ginsburg ME, Sonett JR, et al. Advanced thoracoscopic procedures are facilitated by computer-aided robotic technology. Eur J Cardiothorac Surg 2003;23:883-7; discussion 887. [Crossref] [PubMed]
- Louie BE, Wilson JL, Kim S, et al. Comparison of Video-Assisted Thoracoscopic Surgery and Robotic Approaches for Clinical Stage I and Stage II Non-Small Cell Lung Cancer Using The Society of Thoracic Surgeons Database. Ann Thorac Surg 2016;102:917-24. [Crossref] [PubMed]
- Adams RD, Bolton WD, Stephenson JE, et al. Initial multicenter community robotic lobectomy experience: comparisons to a national database. Ann Thorac Surg 2014;97:1893-8; discussion 1899-900.
- Flores RM, Alam N. Video-assisted thoracic surgery lobectomy (VATS), open thoracotomy, and the robot for lung cancer. Ann Thorac Surg 2008;85:S710-5. [Crossref] [PubMed]
- Oh DS, Reddy RM, Gorrepati ML, et al. Robotic-Assisted, Video-Assisted Thoracoscopic and Open Lobectomy: Propensity-Matched Analysis of Recent Premier Data. Ann Thorac Surg 2017;104:1733-40. [Crossref] [PubMed]
- Melfi FM, Mussi A. Robotically assisted lobectomy: learning curve and complications. Thorac Surg Clin 2008;18:289-95. vi-vii. [Crossref] [PubMed]
- Augustin F, Bodner J, Wykypiel H, et al. Initial experience with robotic lung lobectomy: report of two different approaches. Surg Endosc 2011;25:108-13. [Crossref] [PubMed]
- Veronesi G, Agoglia BG, Melfi F, et al. Experience with robotic lobectomy for lung cancer. Innovations (Phila) 2011;6:355-60. [Crossref] [PubMed]
- Jang HJ, Lee HS, Park SY, et al. Comparison of the early robot-assisted lobectomy experience to video-assisted thoracic surgery lobectomy for lung cancer: a single-institution case series matching study. Innovations (Phila) 2011;6:305-10. [Crossref] [PubMed]
- Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. [Crossref] [PubMed]
- Augustin F, Bodner J, Maier H, et al. Robotic-assisted minimally invasive vs. thoracoscopic lung lobectomy: comparison of perioperative results in a learning curve setting. Langenbecks Arch Surg 2013;398:895-901. [Crossref] [PubMed]
- Cerfolio RJ, Cichos KH, Wei B, et al. Robotic lobectomy can be taught while maintaining quality patient outcomes. J Thorac Cardiovasc Surg 2016;152:991-7. [Crossref] [PubMed]
- Echavarria MF, Cheng AM, Velez-Cubian FO, et al. Comparison of pulmonary function tests and perioperative outcomes after robotic-assisted pulmonary lobectomy vs segmentectomy. Am J Surg 2016;212:1175-82. [Crossref] [PubMed]
- Toker A, Ozyurtkan MO, Kaba E, et al. Robotic anatomic lung resections: the initial experience and description of learning in 102 cases. Surg Endosc 2016;30:676-83. [Crossref] [PubMed]
- Huang J, Li J, Li H, et al. Continuous 389 cases of Da Vinci robot-assisted thoracoscopic lobectomy in treatment of non-small cell lung cancer: experience in Shanghai Chest Hospital. J Thorac Dis 2018;10:3776-82. [Crossref] [PubMed]
- Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg 2011;23:36-42. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Louie BE, Farivar AS, Aye RW, et al. Early experience with robotic lung resection results in similar operative outcomes and morbidity when compared with matched video-assisted thoracoscopic surgery cases. Ann Thorac Surg 2012;93:1598-604; discussion 1604-5. [Crossref] [PubMed]
- Nasir BS, Bryant AS, Minnich DJ, et al. Performing robotic lobectomy and segmentectomy: cost, profitability, and outcomes. Ann Thorac Surg 2014;98:203-8; discussion 208-9. [Crossref] [PubMed]
- Lee BE, Shapiro M, Rutledge JR, et al. Nodal Upstaging in Robotic and Video Assisted Thoracic Surgery Lobectomy for Clinical N0 Lung Cancer. Ann Thorac Surg 2015;100:229-33; discussion 233-4. [Crossref] [PubMed]
- Cerfolio R, Louie BE, Farivar AS, et al. Consensus statement on definitions and nomenclature for robotic thoracic surgery. J Thorac Cardiovasc Surg 2017;154:1065-9. [Crossref] [PubMed]
- Toker A, Kaba E, Ayalp K, et al. Robotic lung resections: video-assisted thoracic surgery based approach. J Vis Surg 2017;3:15. [Crossref] [PubMed]
- Melfi FM, Fanucchi O, Davini F, et al. Robotic lobectomy for lung cancer: evolution in technique and technology. Eur J Cardiothorac Surg 2014;46:626-30; discussion 630-1. [Crossref] [PubMed]