Assessment of chest wall movement following thoracotomy: a systematic review
Introduction
Surgical resection is the mainstay of treatment for early-stage non-small-cell lung cancer and oesophageal cancer (1,2), and the surgical approach can influence the postoperative pulmonary recovery. Traditionally thoracotomy was preferred to minimally invasive surgery (MIS) as it is an established approach with good access and visualisation and improved patient safety particularly during the early stages of surgical training; however, it involves dissection of and trauma to large muscles (3). A study by Nakata et al. [2000] demonstrated the benefits of MIS lobectomy over an open approach on early postoperative pulmonary function preservation (4). Similar observations were reported by a study in 2013 investigating recovery in pulmonary function in the first 3 months after surgery (5).
Previous studies have described the importance of chest wall movement for respiratory function as ventilation becomes dependent on the diaphragm motion when chest expansion is reduced (6). In addition to injury induced by surgical incision and access, abnormal chest wall movement are usually observed following major abdominal surgery due to presence of chest wall oedema, diaphragmatic splinting causing poor respiratory effort resulting in pleural effusion (7). Weakening of chest wall muscles increases the risk for postoperative pulmonary complications (PPC), further impairing the pulmonary function (8).
PPC often arise from multiple peri- and postoperative factors and comprise a wide spectrum of respiratory abnormalities, of which atelectasis, pneumonia and respiratory failure are the most common types (9,10). To date the incidence of PPC following thoracotomy remains high, ranging from 19% to 59% (11,12). Risk factors include cigarette smoking, long duration of anaesthesia, presence of chronic respiratory disease and elderly patients. Hence, patients undergoing thoracic surgery for malignancy are often susceptible for PPC (13).
Chest wall movement and pulmonary function in patients undergoing cardiac surgery was shown to cause asymmetric respiratory movement and a restrictive breathing pattern at 3 months postoperatively when compared with preoperative values (14). Structural abnormalities of the chest wall decrease the chest wall compliance, resulting in an increase in the work of breathing. This affects the lung volumes, resulting in reduced total lung capacity and may also induce changes in the functional residual capacity and residual volume (15-18). To understand the implications of thoracotomy surgery on chest wall function necessities robust methods to quantify chest expansion and breathing abnormalities as a result of changes in chest wall kinematics. The aim of this systematic review is (I) to identify studies assessing changes in chest wall movement using objective measures following thoracotomy, and (II) to assess the clinical relevance of these methods.
Methods
Search strategy and study selection
A literature search was performed to identify relevant studies assessing chest wall movement following thoracotomy in MEDLINE, EMBASE, Cochrane Library, Scopus and Web of Science. The search included the following index or free-text words: ‘thoracotomy’, ‘chest wall’, ‘respiratory mechanics’, ‘inertial measurement unit’, ‘motion capture’, ‘smart textile’, ‘magnetometry’, ‘accelerometry’, ‘photogrammetry’, ‘biosensing techniques’ and ‘plethysmography’. A hand-search was additionally performed to identify missing articles. The full search strategy is shown in Table S1. Two reviewers (Sheraz R. Markar and Karina Tukanova) independently assessed the titles and abstracts for inclusion of relevant references. Articles were included if changes in chest wall movement were evaluated following elective thoracotomy in adult patients using objective measures. Studies were excluded if they assessed chest wall movement following surgery for traumatic injuries. Review articles and case reports were excluded. The following data were extracted: study design, sample size, aim of the study regarding assessment of chest wall mechanics, treatment modality, method of chest expansion measuring and measured parameters, patient acceptability of measuring intervention and the outcome (Table 1).
Full table
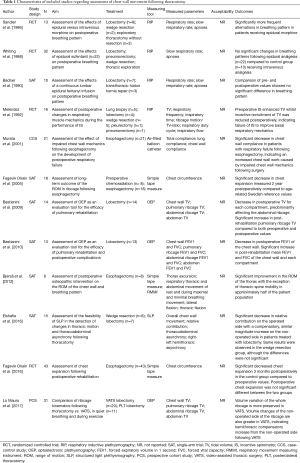
Full table
Quality assessment
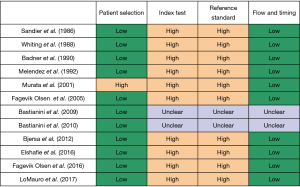
The methodological quality of included studies concerning chest wall movement following thoracotomy was assessed by use of QUADAS-2 tool for evaluation of the risk of primary diagnostic accuracy studies (19). Studies are graded within three domains as ‘high risk’, ‘low risk’ or ‘unclear’.
Results
Literature search results
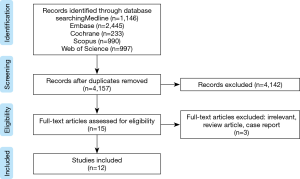
The search for studies assessing chest wall movement yielded 5,791 results. After removal of duplicates, 4,157 references were screened on title and abstract. Fifteen full articles were screened, 12 of which met the inclusion criteria. Selection procedure of the studies is shown in a PRISMA flow diagram (Figure 1) (20).
Twelve studies were reviewed, of which 4 randomized controlled trials (RCT), 1 case-control study (CCS), 1 prospective cohort study (PCS) and 6 single-arm trials (SATs). Included studies are shown in Table 1. Across all studies included, 4 measured the cross-sectional area of the ribcage and abdomen, 1 measured chest wall compliance, 3 measured chest wall circumference and 4 measured chest volume.
Methodological quality
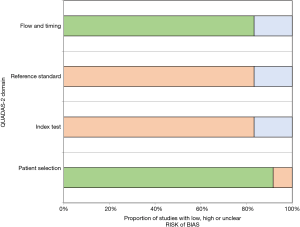
The results of the quality assessment using the QUADAS-2 tool are summarized in Figures 2,3. Only 1 study had a case-control design and the selection of patients could therefore introduce bias. For 10 out of 12 studies, there was no blinding during interpretation of the results of the index test and the reference standard. There was no delay in the collection of data of the index test and reference standard, resulting in a low risk of bias for the flow and timing domain. Across all studies, participants underwent the same reference standard, resulting in a low risk of verification bias.
Measurement of changes in cross-sectional area of the ribcage and abdomen
The first study evaluating breathing movements in patients undergoing thoracotomy, was an RCT conducted by Sandier et al. [1986] (21). Respiratory pattern was assessed with a respiratory inductive plethysmography (RIP) following epidural or intravenous morphine administration. This device allows a non-invasive, continuous monitoring of the respiratory rate and detection of episodes of slow respiratory rate, hypopnoea or apnoea. Compartmental volume changes were measured from variations in cross-sectional areas using 2 bands with incorporated inductive coils. These bands were placed around the ribcage and abdomen, recording the ribcage and abdominal contribution to changes in tidal volume (TV). Ribcage and abdominal motions were captured during tidal breathing in supine and standing positions and calibrated by comparing the output to values obtained from a wedge spirometer. Calibration and validation were repeated in case of an error greater than 10%. Subtle alternations in breathing pattern were observed more frequently in patients receiving epidural morphine (P<0.05), experiencing more often episodes of slow respiratory rate and hypopnoea or apnoea. There was no added value in measuring of the hourly respiratory rate for the assessment of the respiratory pattern. These authors conducted a similar trial to assess the effect of postoperative epidural sufentanil administration (22). The number of apnoeas per hour seemed to be a useful predictor of severe respiratory depression as participants with marked CO2-retention exhibited more than 3 apnoeas per hour on the RIP (P<0.05). Analysis of number of episodes of slow respiratory rate per hour was of limited use. Two years later, the authors conducted a SAT to assess the effect of epidural fentanyl infusion on respiration following thoracotomy. In contrast to previous studies, no significant changes in the respiratory pattern were observed in this study population. Monitoring of respiratory rate also did not appear to be a useful predictor for respiratory depression, in agreement with the findings from previous studies (23).
Melendez et al. [1992] studied changes in respiratory mechanics following thoracotomy using the RIP in patients performing an incentive spirometry (IS) (24). A larger abdominal than ribcage contribution to ventilation was observed in the preoperative setting, whilst postoperative monitoring revealed an altered breathing pattern with increased TV during IS resulting from greater ribcage recruitment. Furthermore, the RIP allowed assessment of the role of recumbency angle, showing greater abdominal motion during postoperative IS at 30° inclination compared to upright position at 60° (P<0.05).
Measurement of chest wall compliance
Murata et al. [2001] computed lung and chest wall compliance to evaluate the contribution of impaired chest wall mechanics following thoracotomy on the development of respiratory failure (25). Intra-pleural pressure was monitored by inserting an air-filled balloon catheter into the pleural cavity and respiratory flow was measured by a flow-calibrated, heated pneumotachograph. Compliance was calculated by the zero-flow method using the latter parameters. This technique allows to detect impairment in respiratory mechanics by calculating the respiratory work of breathing. This was achieved by summing the chest wall and the lung respiratory work, obtained from measuring the pleural pressure and chest wall compliance, respectively. Respiratory mechanics were compared between 2 patient groups, based on either successful or failed weaning from mechanical ventilation. Patients with postoperative failure requiring mechanical ventilation, presented with significantly lower chest wall compliance, indicating a greater respiratory work of breathing in this group (P<0.05). Since the lung respiratory work did not differ significantly in the 2 groups, the increased respiratory muscle energy expenditure could be explained by significant impairment in chest wall mechanics following thoracotomy.
Measurement of chest wall circumference
A practical and cheap measurement tool of the chest wall circumference is by means of a simple tape measure. One RCT and 2 SATs measured the chest circumference (in cm) by computing the difference between maximal expiration and maximal inspiration at the level of the xiphoid. All 3 studies assessed patients who had undergone esophagectomy. Fagevik Olsén et al. [2005] assessed long-term functional outcome at 2 years postoperatively and found significantly decreased chest expansion, compared to the Swedish population norm (P<0.01) (26). In 2016 these authors conducted an RCT evaluating the effect of postoperative rehabilitation on chest expansion and they observed a significantly decreased chest expansion in the control group compared to patients receiving physiotherapy (P<0.05) (27).
In 2012 an article was published by Bjerså et al. in which the effect of osteopathic intervention was assessed on the chest wall motion and breathing pattern, measured by 2 different methods (28). A simple tape measure was used to determine the chest circumference, whilst a respiratory movement measuring instrument (RMMI) computed the anterior-posterior diameter. The latter system measures thoracic and abdominal respiratory motions in rest and during maximal and minimal breathing. Significant improvement in chest expansion could be observed by both measuring methods in approximately of the patients following rehabilitation (P<0.05).
Measurement of chest volume
Respiratory muscle mechanics may be assessed by measuring changes in chest volume with an optoelectronic plethysmography (OEP), also known as structured-light plethysmography. Tidal breathing is analysed for pulmonary ribcage, abdominal ribcage, the abdomen and the torso consisting of these 3 compartments. Furthermore, left and right differences may be evaluated for each of these compartments, allowing detection of asynchrony between the healthy and affected side. Variations in TV are measured by capturing chest wall movement during respiration using light-reflective markers placed on the chest wall surface. The positions of the markers are computed in 3D using stereophotogrammetry with calculation of the volume by connecting the points to form a mesh of triangles. In 2009 Bastianini et al. investigated the usability of OEP as a diagnostic device to assess chest wall motion in patients undergoing lobectomy (29). Chest wall motion was monitored during 2 minutes of tidal breathing and the mean TV was compared in the pre- and postoperative phase and following rehabilitation. Decrease in mean TV was observed across all compartments following surgery, mainly affecting the abdominal part of the ribcage. Respiratory rehabilitation increased the TV in all compartments, with the exception of abdominal ribcage. These post-rehabilitation results did however not reach preoperative values. Hemithoracic analysis revealed that rehabilitation improved the non-operated side of the pulmonary ribcage, compensating for the volume loss of the operated side. One year later the authors used the OEP, measuring the forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) to quantify respiratory impairment following lobectomy and to assess changes following pulmonary rehabilitation (30). A similar trend was observed as in the previous study, demonstrating deterioration in the postoperative measured parameters after which the pulmonary function partially recovered following rehabilitation. These changes in FEV1 and FVC were however more significant than TV variations demonstrated in the previous article. Elshafie et al. [2016] implemented the OEP in patients treated with different types of lung resections. A significant reduction in chest wall motion was observed on the operated side with a compensatory increase on the unaffected side (P=0.01) (31). Furthermore, patients treated with lobectomy revealed significantly greater asynchrony between the operated and healthy thoracic wall than those undergoing wedge resection (P=0.02). A more recent study investigated the impact of the surgical technique on respiratory mechanics using the OEP (32). Comparison of MIS lobectomy with an open approach showed better preservation of ribcage expansion in the former approach. The OEP also enabled the investigators to observe different compensatory changes of chest wall expansion. Ribcage expansion shifted towards the contralateral chest wall in patients undergoing MIS and towards the contralateral abdominal side following open surgery. None of the included studies assessed the patient acceptability of the measuring devices.
Discussion
Chest wall muscles provide protection to vital thoracic structures and contribute to respiration when increased ventilatory effort is required, such as occurs during exercise or the presence of underlying respiratory disease. Chest wall mobility, as measured by thoracic and axillary cirtometry, is related to respiratory muscle strength and lung volumes in healthy subjects. Greater chest expansion was correlated to higher maximal inspiratory and expiratory pressures, FVC, FEV1 and inspiratory capacity (5). Abnormalities such as kyphoscoliosis, pectus excavatum and other disorders affecting the non-muscular structures of the chest wall, decrease the chest wall compliance, resulting in a restrictive pulmonary impairment (33).
Thoracotomy provides excellent exposure and access to vital structures for lung and oesophageal cancer surgical resection. Nevertheless, this surgical intervention leads to a reduction in chest wall compliance caused by distortion in the chest wall configuration (34,35). The respiratory function may additionally be impaired from subsequent chronic post-thoracotomy pain (36-38). Several objective measures have been utilized in measuring chest wall displacement following thoracotomy and all were able to detect changes in patients who had undergone surgery. These methods differed in practical use and invasive nature.
The reliability and accuracy of the simple tape measure has been evaluated in chronic obstructive pulmonary disease (COPD) patients and despite finding high reliability, a high inter-observer variability was observed at all chest wall levels (39). The use of reference tests in chest wall expansion is also subject to potential bias, as it was found that significant differences comparing variations in chest expansion using 2 alternative instructions (40). The use of an air-filled balloon catheter inserted into the pleural space has also been described, this allows assessment of breathing pattern by computing lung and chest wall compliance (25). Although this approach enables to compute lung and chest wall contributions to respiratory work separately, it is not a convenient procedure compared to other methods due to its invasive nature and demand for advanced skills.
Two articles described the implementation of RIP and calibrated the data by comparing measured values with the output obtained from spirometry. Using this method might however induce information bias. Another issue that could affect the validity of this system, is the variation of measurements occurring in different posture as a consequence of changes in ribcage to abdominal contribution (41). OEP is similar device but in contrast to RIP, this is a non-contact system using the position of reflective markers to compute chest wall volume of six different compartments (29). Calibration is obtained through performance of special manoeuvres and by use of spirometry. Other than breathing movements may however induce motion artefacts. Consequently, the same risks of bias apply as with RIP (42). Bastianini et al. [2009] demonstrated the feasibility of OEP to perform compartmental analysis of the TVs of the chest wall and abdomen, which is not possible to achieve using the conventional lung function tests (29). In addition, these authors proved the usability of the system to measure the FEV1 and FVC and found similar results to their first study (30). Hence, this equipment provides a more accurate quantification of the respiratory impairment. The clinical relevance has also been demonstrated by these authors as they assessed chest wall changes following pulmonary rehabilitation, showing partial recovery of the lung volumes. These findings might thus aid in the development of tailored chest physiotherapy following thoracotomy. Furthermore, compartmental analysis of breathing movements with the OEP allows to assess the effect of the amount of lung resected on chest wall movement (31) and the feasibility of this system to evaluate the impact of the surgical approach on respiratory impairment has been established by Lo Mauro et al. (31,32).
Some limitations are present in this review. Chest wall displacement was observed in patients following thoracotomy without comparison to healthy subjects. Furthermore, the vast majority of the papers consisted of a small sample size which might have introduced bias as well. Two studies assessed chest wall movement at several months following surgery and only one study evaluated long-term functional outcomes at 2 years postoperatively. The outcomes may therefore be influenced by pain, PPC and other components in the immediate postoperative phase. None of the studies implemented a measurement standard for chest wall movement as several studies measured the pulmonary function by means of a spirometry and used this as a reference method instead. Finally, six papers were excluded as they were not available in English.
In summary, several objective measures have demonstrated their usability for the assessment of changes in chest wall movement following thoracotomy. Implementation of the type of measuring device should be considered regarding their practical use and invasive nature. Compartmental analysis of the chest wall using the OEP provides a more accurate assessment compared to other systems, allowing evaluation of the impact of the type of surgery on respiratory impairment. Furthermore, this system might aid in the development of tailored physiotherapy following thoracotomy.
Acknowledgments
Funding: Mr. Sheraz R. Markar is funded by the National Institute of Health Research NIHR). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Van Schil PE, Balduyck B, De Waele M, et al. Surgical treatment of early-stage non-small-cell lung cancer. EJC Suppl 2013;11:110-22. [Crossref] [PubMed]
- Ando N, Ozawa S, Kitagawa Y, et al. Improvement in the results of surgical treatment of advanced squamous esophageal carcinoma during 15 consecutive years. Ann Surg 2000;232:225-32. [Crossref] [PubMed]
- Khan IH, McManus KG, McCraith A, et al. Muscle sparing thoracotomy: a biomechanical analysis confirms preservation of muscle strength but no improvement in wound discomfort. Eur J Cardiothorac Surg 2000;18:656-61. [Crossref] [PubMed]
- Nakata M, Saeki H, Yokoyama N, et al. Pulmonary function after lobectomy: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2000;70:938-41. [Crossref] [PubMed]
- Lanza Fde C, de Camargo AA, Archija LR, et al. Chest wall mobility is related to respiratory muscle strength and lung volumes in healthy subjects. Respir Care 2013;58:2107-12. [Crossref] [PubMed]
- Grimby G, Fugl-Meyer AR, Blomstrand A. Partitioning of the contributions of rib cage and abdomen to ventilation in ankylosing spondylitis. Thorax 1974;29:179-84. [Crossref] [PubMed]
- Katz JA, Zinn SE, Ozanne GM, et al. Pulmonary, chest wall, and lung-thorax elastances in acute respiratory failure. Chest 1981;80:304-11. [Crossref] [PubMed]
- Lunardi AC, Miranda CS, Silva KM, et al. Weakness of expiratory muscles and pulmonary complications in malnourished patients undergoing upper abdominal surgery. Respirology 2012;17:108-13. [Crossref] [PubMed]
- Sengupta S. Post-operative pulmonary complications after thoracotomy. Indian J Anaesth 2015;59:618-26. [Crossref] [PubMed]
- Agostini P, Lugg ST, Adams K, et al. Postoperative pulmonary complications and rehabilitation requirements following lobectomy: a propensity score matched study of patients undergoing video-assisted thoracoscopic surgery versus thoracotomy. Interact Cardiovasc Thorac Surg 2017;24:931-7. [Crossref] [PubMed]
- García-Miguel FJ, Serrano-Aguilar PG, López-Bastida J. Preoperative assessment. Lancet 2003;362:1749-57. [Crossref] [PubMed]
- Gracey DR, Divertie MB, Didier EP. Preoperative pulmonary preparation of patients with chronic obstructive pulmonary disease: a prospective study. Chest 1979;76:123-9. [Crossref] [PubMed]
- Rock P, Rich P. Postoperative pulmonary complications. Curr Opin Anaesthesiol 2003;16:123-31. [Crossref] [PubMed]
- Kristjánsdóttir A, Ragnarsdóttir M, Hannesson P, et al. Respiratory movements are altered three months and one year following cardiac surgery. Scand Cardiovasc J 2004;38:98-103. [Crossref] [PubMed]
- Vallbona C, Spencer WA. The total lung capacity and its subdivisions. J Chronic Dis 1959;9:617-35. [Crossref] [PubMed]
- Hart N., Cramer D, Ward SP, et al. Effect of pattern and severity of respiratory muscle weakness on carbon monoxide gas transfer and lung volumes. Eur Respir J 2002;20:996-1002. [Crossref] [PubMed]
- Hopkins E, Sharma S. Physiology, functional residual capacity. Treasure Island: StatPearls Publishing, 2019.
- George RB, Light RW, Matthay MA, et al. Chest medicine: essentials of pulmonary and critical care medicine. 5th ed. New York: Lippincott Williams & Wilkins, 2005.
- Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. [Crossref] [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [Crossref] [PubMed]
- Sandler AN, Chovaz P, Whiting W. Respiratory depression following epidural morphine: a clinical study. Can Anaesth Soc J 1986;33:542-9. [Crossref] [PubMed]
- Whiting WC, Sandler AN, Lau LC, et al. Analgesic and respiratory effects of epidural sufentanil in patients following thoracotomy. Anesthesiology 1988;69:36-43. [Crossref] [PubMed]
- Badner NH, Sandler AN, Koren G, et al. Lumbar epidural fentanyl infusions for post-thoracotomy patients: analgesic, respiratory, and pharmacokinetic effects. J Cardiothorac Anesth 1990;4:543-51. [Crossref] [PubMed]
- Melendez JA, Alagesan R, Reinsel R, et al. Postthoracotomy respiratory muscle mechanics during incentive spirometry using respiratory inductance plethysmography. Chest 1992;101:432-6. [Crossref] [PubMed]
- Murata K, Kubota T. Impairment of chest wall mechanics and increased chest wall work of breathing cause postoperative respiratory failure in patients who have undergone radical esophagectomy. J Anesth 2001;15:125-31. [Crossref] [PubMed]
- Fagevik Olsén M, Larsson M, Hammerlid E, et al. Physical function and quality of life after thoracoabdominal oesophageal resection: results of a longitudinal follow-up study. Dig Surg 2005;22:63-8. [Crossref] [PubMed]
- Fagevik Olsén M, Kjellby Wendt G, Hammerlid E, et al. Effects of a training intervention for enhancing recovery after ivor-lewis esophagus surgery: a randomized controlled trial. Scand J Surg 2017;106:116-25. [Crossref] [PubMed]
- Bjerså K, Sachs C, Hyltander A, et al. Osteopathic intervention for chronic pain, remaining thoracic stiffness and breathing impairment after thoracoabdominal oesophagus resection: a single subject design study. Int J Osteopath Med 2013;16:68-80. [Crossref]
- Bastianini F, Silvestri S, Magrone G, et al. A preliminary efficacy evaluation performed by opto-electronic plethysmography of asymmetric respiratory rehabilitation. Conf Proc IEEE Eng Med Biol Soc 2009;2009:849-52.
- Bastianini F, Silvestri S, Schena E, et al. Evaluation of pulmonary rehabilitation after lung resection through opto-electronic plethysmography. Conf Proc IEEE Eng Med Biol Soc 2010;2010:2481-4.
- Elshafie G, Kumar P, Motamedi-Fakhr S, et al. Measuring changes in chest wall motion after lung resection using structured light plethysmography: a feasibility study. Interact Cardiovasc Thorac Surg 2016;23:544-7. [Crossref] [PubMed]
- Mauro AL, Palleschi A, Privitera E, et al. Ribcage kinematics during exercise justifies thoracoscopic over thoracotomy lobectomy prompt recovery. Eur Respir J 2016;48:OA1988.
- Tzelepis GE. Chest wall diseases respiratory pathophysiology. Clin Chest Med 2018;39:281-96. [Crossref] [PubMed]
- Siafakas NM, Mitrouska I, Bouros D, et al. Surgery and the respiratory muscles. Thorax 1999;54:458-65. [Crossref] [PubMed]
- Karlson KE, Seltzer B, Lee S, et al. Influence of thoracotomy on pulmonary mechanics: association of increased work of breathing during anesthesia and postoperative pulmonary complications. Ann Surg 1965;162:973-80. [Crossref] [PubMed]
- Rogers ML, Duffy JP. Surgical aspects of chronic post-thoracotomy pain. Eur J Cardiothorac Surg 2000;18:711-6. [Crossref] [PubMed]
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618-25. [Crossref] [PubMed]
- Mesbah A, Yeung J, Gao F. Pain after thoracotomy. BJA Educ 2016;16:1-7. [Crossref]
- Malaguti C, Rondelli RR, de Souza LM, et al. Reliability of chest wall mobility and its correlation with pulmonary function in patients with chronic obstructive pulmonary disease. Respir Care 2009;54:1703-11. [PubMed]
- Olsén MF, Lindstrand H, Broberg JL, et al. Measuring chest expansion; a study comparing two different instructions. Adv Physiother 2011;13:128-32. [Crossref]
- Stradling JR, Chadwick GA, Quirk C, et al. Respiratory inductance plethysmography: calibration techniques, their validation and the effects of posture. Bull Eur Physiopathol Respir 1985;21:317-24. [PubMed]
- Parreira VF, Vieira DS, Myrrha MA, et al. Optoelectronic plethysmography: a review of the literature. Rev Bras Fisioter 2012;16:439-53. [Crossref] [PubMed]