The European experience
Introduction
The first anatomical lung segmentectomy was performed over 80 years ago for bronchiectasis in the lingula (1). Curiously, pneumonectomy was considered the gold standard for the treatment of lung cancer at the time (2). The progressive increase in the detection of small lung tumours, as well as ground glass opacities (GGOs), pushed surgeons to carry out limited pulmonary resections, in order to preserve lung tissue. In 1995, the Lung Cancer Study Group (LCSG) demonstrated a higher rate of local recurrences in patients treated with limited resections (3). Despite the bias in this study, such as the small sample size and the inclusion of tumours up to 3cm in size, the western world grew sceptical towards sublobar resections, and lobectomy became the treatment of choice for lung cancer. Until 2000, very few articles regarding sublobar resections have been published in western countries, and these works were mostly little case series (4-6). These procedures, in fact, were usually offered to elderly patients (7), metastatic cancer patients (8) or to those with a second primary lung cancer (9-11). In this article we debate the current trends in the treatment of early stage non-small cell lung cancer (NSCLC) in Europe.
Patients’ selection and choice of the procedure
Historically, limited resections for stage I–II lung cancer were offered to patients with compromised respiratory function or severe cardiovascular comorbidities. In a retrospective study on 472 patients with stage I NSCLC, Pastorino et al. reported 61 sublobar resections (wedge and segmentectomies); in this group of patients, 50% had cardio-respiratory comorbidities. The Authors concluded that a more conservative treatment should be considered in patients with severe concurrent pathologies (4).
In 2004, Martin-Ucar et al. published a retrospective study on anatomical segmentectomies in a high risk population: patients undergoing this procedure had a mean FEV1 of 43.7% and a mean ppoFEV1 of 32.6%. Every patient from this group was individually matched to patients with the same characteristics who underwent pulmonary lobectomy. The Authors asserted that anatomical resection in patients with early stage NSCLC and a poor respiratory function was feasible, with good outcomes in the long term (12).
A few years later, Gossot et al. presented the initial results of a study including patients who underwent totally endoscopic major pulmonary resection for first stage NSCLC. In this work, anatomical sublobar resections were reserved to patients with an impaired respiratory function that would have made lobectomy a high-risk procedure (13).
Witte et al. too reported complete video-assisted thoracoscopic surgery (VATS) segmentectomies in a single-centre prospective observational study including 20 consecutive patients with clinical stage I lung carcinoma. They recommended sublobar resection in those patients with at least one contraindication to lobectomy, generally referring to severe comorbidities and poor respiratory function (14).
In this scenario, patients' age has always been a key factor: many surgeons considered sublobar resection as the most appropriate approach in elderly patients. An Italian multicentre study by Fiorelli et al. retrospectively considered 239 patients over 75 years undergoing surgery for early stage NSCLC (i.e., T1a,b-2aN0). In 149 (62.3%) patients, lobectomy was performed, while 90 patients underwent sublobar resection (39 segmentectomies, 51 wedge resections). The latter had a significantly worse preoperative and predictive postoperative respiratory function, an impaired cardiac function and a higher Charlson comorbidity index. Based on the analyses of the oncological outcomes, the authors inferred that compromised elderly patients could benefit from lung-sparing procedures (15).
In the last few years, as shown by the Literature, anatomical segmentectomy has been increasingly performed intentionally, namely in patients with a small primary tumour who could endure lobectomy. This trend was a consequence of both the increase in diagnosis of small lesions, also thanks to screening programs (16,17), and the publication of various studies showing that anatomical segmentectomy was an adequate approach for tumours smaller than 2 cm (18).
However, the adoption rate of this technique was quite heterogeneous among different countries around Europe: Petersen presented the data from the Danish Lung Cancer Screening, which included only one segmentectomy out of 49 operations, 37 of those being major resections performed by VATS. In this study, lobectomy was considered the gold standard approach (19). In the Dutch-Belgian Nelson lung cancer screening trial, only 4 segmentectomies have been reported out of 198 operations (20).
On the other side, Infante et al. published the surgical results from the DANTE Trial screening program: elective anatomical segmentectomy was performed in 19% of the cases, in patients with both a poor respiratory function and small peripheral solid lesions and/or GGOs. The Authors argued that in patients with screening-detected lesions, segmentectomy would often be an adequate choice, as it allows local control of the disease with a limited reduction of respiratory function. Nonetheless, segmentectomy remains a technically challenging procedure that necessitates adequate training for the surgical team. Therefore, surgeons need time to overcome the appropriate learning curve, particularly when referring to VATS technique: out of the 11 segmentectomies performed, 3 underwent reoperation (one for bleeding, the other two because of venous infarction of the lingula after culmenectomy) (21).
Morgant reported a study regarding time trends in the surgical treatment of lung cancer in France: by extrapolated data from the Epithor clinical database, he detected an increase in the number of early stage NSCLC diagnosis. This caused a reduction in the number of pneumonectomies performed compared to lobectomies, but most of all it determined a significant increase in the number of anatomical segmentectomies, which went from 2.6% in 2005–2006 to 5.4% in 2011–2012 (22).
Gossot’s research team recently published the data on survival in 648 patients undergone totally endoscopic major pulmonary resection for I stage NSCLC from 2007 to 2016. The Authors underline that with change in patients’ characteristics, sublobar resection increased from 8% in 2007–2008 to 37.6% in 2016; in this study, segmentectomy was offered to patients with nodules <2 cm and to patients with cT1b cancer who had had a prior major pulmonary resection and/or had an impairment in respiratory function (23).
Type of surgical approach
Over the past three decades, the pursuing of progressively less invasive surgical approaches radically changed the way pulmonary resections are performed. As it was for lobectomies, anatomical segmentectomies were historically carried out via thoracotomy. In 2008, Sienel et al. published a study to compare wedge resections and segmentectomies: all the 56 segmentectomies described where performed by posterolateral or anterolateral muscle sparing thoracotomy (24). In time, though, VATS approach gained increasing popularity around Europe and surgeons started to apply this technique to segmentectomies. However different centres around Europe still adopt various techniques.
VATS technique for anatomical segmentectomies has been shown to be safe and feasible by different studies (12,23,25). Even though it requires more technical precision and awareness of the anatomy of the lungs, VATS segmentectomy has also been associated with a reduced post-operative mortality (25), similar postoperative complications and reduced length of stay, when compared to thoracotomic segmentectomy (26).
In 2011, Gossot et al. published the initial data regarding a totally endoscopic approach (i.e., four trocars, no access incision) for anatomical segmentectomies, including 50 patients; in 20 of those cases, the procedure was associated with radical lymphadenectomy (13). Witte too reported his experience on complete VATS segmentectomies in a case series including 20 consecutive anatomical sublobar resections: her preferred approach consisted of a three-portal VATS (one anterior 2-4cm utility incision and two epiphrenic trocars), and lymph-nodes dissection was carried out in a systematic way (14). In another work published by the same Authors in 2015, reporting their experience from 2002 to 2012, they described 100 anatomical sublobar resections: 56 of them were carried out by three-portal VATS and 44 by thoracotomy. When comparing those two groups, the Authors found that VATS approach was associated with a reduced length of hospital stay and less post-operative comorbidities; moreover, VATS technique resulted slightly better in terms of five-years overall survival and recurrence-free survival (27).
In the same period, different centres were still performing mostly open surgery. In 2011, in Italy, the data by Infante reporting the experience of the DANTE trial showed lateral thoracotomy and posterolateral thoracotomy as the routinely used approach; among those procedures, only one VATS segmentectomy was performed (21).
When analysing the results of the Danish Lung Cancer Screening Trial published by Petersen in 2012, 84% out of 49 operations were carried out by VATS, but only one of them was an anatomical segmentectomy (19). Dziedzic collected and published data from the Polish National Lung Cancer Registry: 23 segmentectomies were performed from 2007 to 2013, 13 of them (5.6%) via VATS (28).
In 2012, Gonzalez-Rivas reported the first anatomical segmentectomy performed by uniportal VATS, showing the feasibility of this complex procedure, even with the utmost minimally invasive approach described (29).
In time, following the progressive worldwide diffusion of uniportal VATS for lobectomies, experienced surgeons started to apply this technique for more demanding procedures such as anatomical segmentectomies.
In 2017, Surendrakumar reported 86 consecutive patients undergone segmentectomy in a 36 months period: 52 resections where performed via VATS (73% of them being uniportal). In this study, survival, complications and readmissions rate were similar after open and VATS segmentectomy; the second group showed a shorter length of stay and a shorter chest tube placement duration. Moreover, no difference between the two groups was observed in the number of lymph nodes resected (26).
Only few data are available regarding robotic segmentectomies: in 2012, Pardolesi reported 17 patients who underwent robotic segmentectomy with Da Vinci System in two centres. The approach consisted of 3 or 4 ports, depending on the surgeon performing the segmentectomy. The Authors claim that this technique is feasible and safe, and that robotic surgery has several theoretical advantages over VATS surgery, such as a three dimensional field of view and the absence of the fulcrum effect (30). In order to further move towards minimally invasive techniques, some centres are starting to adopt non-intubated VATS segmentectomies (NITS): in 2014, Mineo described 36 cases undergoing NITS for stage I lung cancer: the rate of conversion to general anesthesia was 2.8%, and he observed a reduction in the length of stay and in the morbidity rate compared to the same procedure performed with general anesthesia. The author recommends a thorough study of the upper airways in the event of unexpected events during the procedure (31).
Oncological outcomes
The oncological view should be the first point of interest when arguing about tissue-sparing oncologic surgery. It is interesting to notice that among 38 final selected articles in this review article, only 16 adequately cover the oncological aspect of segmentectomy in early stage lung cancer.
In 2004, an Italian retrospective analysis did not show any difference in survival after lobectomy vs. segmentectomy for stage I NSCLC; on the contrary, a higher incidence of local recurrence in segmentectomy was noticed (32). It is well known that when comparing different types of sublobar resections, segmentectomy is superior to wedge, as confirmed by Sienel et al. in a retrospective analysis of sublobar resection in compromised patients (24). Sienel himself previously focused the attention on the local recurrence rate, stating that it is, of course, higher in segmentectomy than lobectomy, but depends on the different segments resected, recommending to avoid segmentectomy in S1-3 region (33). Martin-Ucar et al., in a case-matched analysis of high-risk stage I NSCLC did not see any difference both in local recurrence rate and survival between lobectomy and segmentectomy (12). The Italian DANTE trial established that minimally invasive approach and lung-sparing procedures, such as segmentectomies, could lead to achieve local control and should be used in screened-derived patients, that more often undergo surgery for benign disease (21). Once more in Italy, Mattioli et al. in a case-match study comparing 46 lobectomy with 46 segmentectomy for cT1aN0M0 lung cancer, reported no differences in the number of N1 or N2 lymph nodes resected; also, the two groups had a similar cancer-specific survival (34). In 2015 Witte et al. focused on surgical approach to segmentectomy, comparing thoracotomy to VATS, concluding that thoracoscopic access is probably not inferior to thoracotomy in long-term oncological outcome (27). An Italian retrospective, multicenter study, analysing a high-risk elderly population affected by stage I NSCLC, showed no differences between lobectomies and sublobar resections (comparing both segmentectomy and wedge resections) in terms of long-term survival (15). A polish group published in 2017 a national lung cancer registry-based analysis of lobectomy vs. segmentectomy for stage I NSCLC with no differences in 3-year or 5-year survival rates (28); similar results have been observed by a propensity score matched analysis published in 2019 by Roman et al., with no differences in 5-year survival between lobectomy and segmentectomy for stage I tumors (35).
Discussion
Historically, pulmonary lobectomy has always been considered the procedure of choice for the treatment of lung cancer, even for early stages. Surgeons tended to think of limited pulmonary resection as an option only for patients with a poor cardiorespiratory function (4,12-14), elderly patients (15) or patients with a second primary lung cancer (9-11).
The only randomized clinical trial, published by Ginsberg in 1995, enrolled patients with cT1N0M0 NSCLC: in this study, 122 patients undergone sublobar resections were compared to 125 lobectomies. The results showed an increased mortality rate in the limited resection group, and the locoregional disease recurrence was about three times higher than that for the lobectomy group. However, it should be underlined that, in this study, the nodules’ maximum diameter included was 3 cm (3).
Other data regarding oncological outcomes after anatomical segmentectomies come exclusively from retrospective works; the lack of randomized trials showing similar results in overall survival between sublobar resections and lobectomy produced growing scepticism among surgeons.
Nonetheless, from the beginning of 2000, the number of small lung tumours detected started to significantly increase, also due to low-dose CT screening programs carried out in different European countries (Netherlands-Belgium, UK, Denmark, Italy, Germany, Poland, Spain, Switzerland). The percentage of lung cancer lesions diagnosed at an early stage (i.e., stage I and II) with those programs were 80% (36), while only 16% were at an early stage among the non-screened population.
Furthermore, the development of high-resolution CTs increased the diagnosis of GGOs, lesions with a heterogeneous biologic behaviour that are potentially indolent or low malignant (37).
In the same period, various studies carried out mostly in Asian countries reported similar oncological outcomes in patients with lesions up to 2cm who underwent segmentectomies or lobectomies.
In a multicentre trial including 3 Japanese Hospitals and 567 low-risk patients with clinical T1N0M0 peripheral tumours up to 2 cm, 305 (53.8%) underwent sublobar resection while the other 262 underwent lobectomy: the results showed no differences between the two groups in terms of disease-free survival and overall survival. This study emphasized the importance of the intraoperative confirmation of the stage by frozen-section analyses of sampled lymph nodes (segmental, hilar, mediastinal) (18).
Martin-Ucar too, in a retrospective study on 55 NSCLC stage I high-risk patients with respiratory comorbidities, declared that the oncological efficacy of limited resections was similar to that of lobectomies (12).
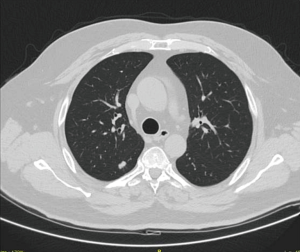
In the face of this favourable data on survival and considering the increased detection of lung nodules with no diagnosis, the interest of surgeons towards more conservative procedures began to grow. The combination of a minimally-invasive surgical approach (i.e., VATS, RATS) and a lung-tissue sparing procedure is by now considered the best therapeutic choice in selected patients with stage Ia lung cancer located in the outer third of the lung (Figure 1).
To this day, the recommendations from the European Society of Thoracic Surgeons (ESTS) on CT screening are in favour of this approach, claiming that in case of suspicious lung lesions up to 2 cm and with no diagnosis that are entirely resectable by anatomical segmentectomy, performing a minimally invasive segmentectomy with a diagnostic and therapeutic aim can be acceptable (38).
Since segmentectomy is a technically demanding procedure, the need for an adequate learning curve is most certainly an obstacle to its worldwide diffusion: the main challenges are represented by the correct identification of the intersegmental plane and the segmental lymphadenectomy, the latter being mandatory in this particular operation.
Because of these reasons, some anatomical sublobar resections are carried out more frequently than others (i.e., apical segment of the lower lobes, lingulectomy, left apical trisegmentectomy), and they are performed only by surgeons with a lot of experience, especially by VATS (39). Scientific Literature, however, shows an increasing spread of anatomical segmentectomies around Europe, which leads us to think that, as it happened before for other types of cancer, this most conservative approach is bound to become the procedure of choice in selected patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mario Nosotti, Ilaria Righi and Lorenzo Rosso) for the series “Early Stage Lung Cancer: Sublobar Resections are a Choice?” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.01.26). The series “Early Stage Lung Cancer: Sublobar Resections are a Choice?” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Churchill ED, Belsey R. Segmental pneumonectomy in bronchiectasis. Ann Surg 1939;109:481-99. [Crossref] [PubMed]
- Horn L, Johnson DH, Evarts A. Graham and the first pneumonectomy for lungcancer. J Clin Oncol 2008;26:3268-75. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Pastorino U, Valente M, Bedini V, et al. Limited resection for Stage I lung cancer. Eur J Surg Oncol 1991;17:42-6. [PubMed]
- Borrelly J, Martin F, Grosdidier G, et al. Value of limited resections in the surgical treatment of lung cancers. Ann Chir 1992;46:766-9. [PubMed]
- Motta G, Nahum MA, Testa T, et al. Resection for peripheral higher stage lung tumor. A compared Italian cumulative experience. Ann Ital Chir 1996;67:381-5. [PubMed]
- Loizzi M, Sardelli P, Sollitto F, et al. Lung resections for cancer in the elderly. Minerva Chir 1998;53:489-95. [PubMed]
- Venn GE, Sarin S, Goldstraw P. Survival following pulmonary metastasectomy. Eur J Cardiothorac Surg 1989;3:105-9; discussion 110. [Crossref] [PubMed]
- Spaggiari L, Grunenwald D, Girard P, et al. Cancer resection on the residual lung after pneumonectomy for bronchogenic carcinoma. Ann Thorac Surg 1996;62:1598-602. [Crossref] [PubMed]
- Voltolini L, Paladini P, Luzzi L, et al. Iterative surgical resections for local recurrent and second primary bronchogenic carcinoma. Eur J Cardiothorac Surg 2000;18:529-34. [Crossref] [PubMed]
- Zuin A, Andriolo LG, Marulli G, et al. Is lobectomy really more effective than sublobar resection in the surgical treatment of second primary lung cancer? Eur J Cardiothorac Surg 2013;44:e120-5; discussion e125.
- Martin-Ucar AE, Nakas A, Pilling JE, et al. A case-matched study of anatomical segmentectomy versus lobectomy for stage I lung cancer in high-risk patients. Eur J Cardiothorac Surg 2005;27:675-9. [Crossref] [PubMed]
- Gossot D, Girard P, Raynaud C, et al. Fully endoscopic major pulmonary resection for stage I bronchial carcinoma: initial results. Rev Mal Respir 2011;28:e123-30. [Crossref] [PubMed]
- Witte B, Wolf M, Hillebrand H, et al. Complete video-assisted thoracoscopic surgery anatomic segmentectomy for clinical stage I lung carcinoma - technique and feasibility. Interact Cardiovasc Thorac Surg 2011;13:148-52. [Crossref] [PubMed]
- Fiorelli A, Caronia FP, Daddi N, et al. Sublobar resection versus lobectomy for stage I non-small cell lung cancer: an appropriate choice in elderly patients? Surg Today 2016;46:1370-82. [Crossref] [PubMed]
- Infante M, Lutman FR, Cavuto S, et al. Lung cancer screening with spiral CT: baseline results of the randomized DANTE trial. Lung Cancer 2008;59:355-63. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Petersen RH, Hansen HJ, Dirksen A, et al. Lung cancer screening and video-assisted thoracic surgery. J Thorac Oncol 2012;7:1026-31. [Crossref] [PubMed]
- Van't Westeinde SC, Horeweg N, De Leyn P, et al. Complications following lung surgery in the Dutch-Belgian randomized lung cancer screening trial. Eur J Cardiothorac Surg 2012;42:420-9. [Crossref] [PubMed]
- Infante M, Chiesa G, Solomon D, et al. Surgical procedures in the DANTE trial, a randomized study of lung cancer early detection with spiral computed tomography: comparative analysis in the screening and control arm. J Thorac Oncol 2011;6:327-35. [Crossref] [PubMed]
- Morgant MC, Pagès PB, Orsini B, et al. Epithor project (French Society of Thoracic and Cardiovascular Surgery). Time trends in surgery for lung cancer in France from 2005 to 2012: a nationwide study. Eur Respir J 2015;46:1131-9. [Crossref] [PubMed]
- Lutz JA, Seguin-Givelet A, Grigoroiu M, et al. Oncological results of full thoracoscopic major pulmonary resections for clinical Stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2019;55:263-70. [Crossref] [PubMed]
- Sienel W, Dango S, Kirschbaum A, et al. Sublobar resections in stage IA non-small cell lung cancer: segmentectomies result in significantly better cancer-related survival than wedge resections. Eur J Cardiothorac Surg 2008;33:728-34. [Crossref] [PubMed]
- Linden D, Linden K, Oparka J. In patients with resectable non-small-cell lung cancer, is video-assisted thoracoscopic segmentectomy a suitable alternative to thoracotomy and segmentectomy in terms of morbidity and equivalence of resection? Interact Cardiovasc Thorac Surg 2014;19:107-10. [Crossref] [PubMed]
- Surendrakumar V, Martin-Ucar AE, Edwards JG, et al. Evaluation of surgical approaches to anatomical segmentectomies: the transition to minimal invasive surgery improves hospital outcomes. J Thorac Dis 2017;9:3896-902. [Crossref] [PubMed]
- Witte B, Stenz C, Vahl CF, et al. Comparative intention-to-treat analysis of the video-assisted thoracoscopic surgery approach to pulmonary segmentectomy for lung carcinoma. Interact Cardiovasc Thorac Surg 2015;21:276-83. [Crossref] [PubMed]
- Dziedzic R, Zurek W, Marjanski T, et al. Stage I non-small-cell lung cancer: long-term results of lobectomy versus sublobar resection from the Polish National Lung Cancer Registry. Eur J Cardiothorac Surg 2017;52:363-9. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Mendez L, et al. Single-port video-assisted thoracoscopic anatomic segmentectomy and right upper lobectomy. Eur J Cardiothorac Surg 2012;42:e169-71. [Crossref] [PubMed]
- Pardolesi A, Park B, Petrella F, et al. Robotic anatomic segmentectomy of the lung: technical aspects and initial results. Ann Thorac Surg 2012;94:929-34. [Crossref] [PubMed]
- Mineo TC, Tacconi F, Ambrogi V, et al. Nonintubated VATS segmentectomy: when and for whom? Ann Thorac Surg 2014;98:388. [Crossref] [PubMed]
- Campione A, Ligabue T, Luzzi L, et al. Comparison between segmentectomy and larger resection of stage IA non-small cell lung carcinoma. J Cardiovasc Surg (Torino) 2004;45:67-70. [PubMed]
- Sienel W, Stremmel C, Kirschbaum A, et al. Frequency of local recurrence following segmentectomy of stage IA non-small cell lung cancer is influenced by segment localisation and width of resection margins--implications for patient selection for segmentectomy. Eur J Cardiothorac Surg 2007;31:522-7; discussion 527-8. [Crossref] [PubMed]
- Mattioli S, Ruffato A, Puma F, et al. Does anatomical segmentectomy allow an adequate lymph node staging for cT1a non-small cell lung cancer? J Thorac Oncol 2011;6:1537-41. [Crossref] [PubMed]
- Roman M, Labbouz S, Valtzoglou V, et al. Lobectomy vs. segmentectomy. A propensity score matched comparison of outcomes. Eur J Surg Oncol 2019;45:845-50. [Crossref] [PubMed]
- International Early Lung Cancer Action Program Investigators, Henschke CI, Yankelevitz DF, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763-71. [Crossref] [PubMed]
- Migliore M, Fornito M, Palazzolo M, et al. Ground glass opacities management in the lung cancer screening era. Ann Transl Med 2018;6:90. [Crossref] [PubMed]
- Pedersen JH, Rzyman W, Veronesi G, et al. Recommendations from the European Society of Thoracic Surgeons (ESTS) regarding computed tomography screening for lung cancer in Europe. Eur J Cardiothorac Surg 2017;51:411-20. [PubMed]
- Mendogni P, Tosi D, Rosso L, et al. VATS segmentectomy: an underused option? J Vis Surg 2017;3:136. [Crossref] [PubMed]