
What is the strategy for strategic arch resection in acute proximal aortic dissection?
Type A aortic dissections are one of the major surgical challenges cardiac surgeons are faced with in their day to day practice. Repair of acute Type A dissections are technically challenging and time consuming and to make it even worse, for unknown reasons, in most cases, happen at night while lasting into the early hours of the new day. It is common practice that these complex operations are performed by the surgical on-call team under sub-optimal conditions at night and not by trained specialist Aortic Surgeons during daytime. As a result of this dilemma the usual approach to the repair of an acute Type A dissection is to get the patient alive out of theatre and do the minimum repair that is required, which in most cases is a supra-commissural replacement of the ascending aorta with an open anastomosis to the proximal aortic arch (often called hemi-arch replacement). It is well established that this surgical approach, while being a rescue undertaking in the acute setting of Type A aortic dissection, leaves a large number of patients with dissected aortic arches, descending and abdominal aortas necessitating further future interventions. As a result the extent of surgery with regards to the aim of a definitive repair involving arch reconstruction/replacement versus a “lifesaving” limited approach, possibly at the expense of a later need for a second intervention such as extensive reconstructive surgery of the arch and adjacent vascular structures is still subject to ongoing debate.
To make their point, the authors present data from a retrospective cohort analysis on outcomes of different surgical strategies for acute type A aortic dissection over a time period of 21 years in a single centre. The authors report that the comparison of a cohort of 322 cases undergoing ascending/hemi-arch replacement (a so-called limited surgical approach) with another cohort of 150 cases undergoing aggressive aortic arch replacement with arch expansion or nonresectable tears—collected from 1996 to 2017—revealed no significant differences in perioperative outcomes and showed a 30-day mortality of 5.3% versus 7.3%, P=0.38, and a intra/post-operative stroke rate of 7% each (1). Even at long-term over 5 years the estimated survival was similar with between the limited resection at 70% and the more aggressive arch repair at 72% (1). Similarly, the annual incidence rate of reoperations over 15 years was 2.1% and 2.0%, P=1.0, and the 10 years cumulative rate of reoperations was 14% versus 12%, P=0.89, without any difference. Hence, based on those figures the authors concluded that ascending/hemi-arch and aggressive total arch replacement are both appropriate in patients in the acute setting of proximal aortic dissection. Subsequently, extended surgery or aggressive arch replacement should be considered in the setting of an aortic arch with a diameter >4 cm or an intimal tear not resected by a hemi-arch approach or in presence of any arch branch malperfusion.
The authors ought to be congratulated to such excellent outcome results with mortality in the single digits regardless of the extent of acute surgery. Unresolved issues and critical questions, however, do remain when comparing outcomes of those two cohorts retrospectively collected over 21 years. The subject Late outcomes of strategic arch resection in acute type A dissection maybe cleverly chosen to describe the authors’ analysis, but all data produced in their analysis fail to answer the question: what to do in a given case of type A aortic dissection in the middle of the night, or as a routine! Moreover, the title may in fact insinuate that anything goes as long as you follow a strategy regardless of the individual expertise of the operator in charge and his or her team, as the aspect of training and expertise has not been addressed at all. Such insinuation would most likely convey the wrong message. Most importantly, by nature cohort studies may at best generate ideas to be tested in carefully designed randomized trials (unlikely be possible in this setting) or in propensity score matched studies (requiring well organized multicentre prospective registries).
Let us have a more detailed and granular look at the data. A closer look reveals differences between both cohorts with regards to demographics and peri-operative characteristics. Interestingly, on top of the different cohort sizes, patients subjected to the aggressive arch repair/replacement were significantly younger at 57 years, had in only 9% associated coronary disease or an acute myocardial infarction (1%), and in only 3% a bicuspid aortic valve (P=0.009). Of patients subjected to the extended operation only 4% had a pericardial tamponade before surgery versus 11% (P=0.01) in the group of ascending/hemi-arch repair; this helps explain that 21% of cases undergoing extensive arch repair/replacement could be performed in a delayed (elective) setting while in the other group just 15% were managed electively and could be deferred (P=0.08). In other words, prognostically important characteristics such as age, hemodynamic stability, and associated vascular/coronary pathology were in favour of patients subjected to the more aggressive surgical approach reflecting some sort of selection process with the idea to offer extensive arch replacement to the “healthier or fitter” patient in the setting of a life-threatening condition to begin with. Moreover, in a granular view on intra- and peri-operation outcomes the interpretation by the authors leave a couple of questions unanswered: How does stroke rate and survival compare and was mortality dominated by the occurrence of stroke in 7% in both groups? Well, the older hemi-arch group with an age of 61 years had a higher incidence of atrial fibrillation in 39% which may have contributed to the 7% stroke rate; thus, without the element of atrial fibrillation stroke rate would be most likely lower with the less aggressive surgical approach. On another note, it is not clear whether there is a hidden selection bias in the sense that some patients with malperfusion syndromes were subjected to a staged strategy with primary interventions to abolish or mitigate malperfusion by stenting or fenestration manoeuvres prior to deferred and then extensive surgery? It is likely that once stabilised those patients could be offered a more extensive surgery in a deferred semi-elective scenario allowing for better planning and avoiding some of the risks of an emergent surgical approach. A simplistic conclusion could be that a fitter patient has a better chance to survive, which is no news!
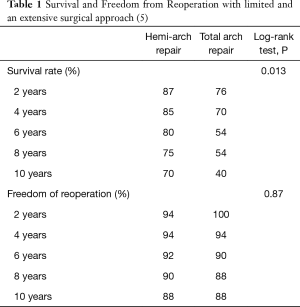
A close look at the intraoperative data reveals some idiosyncrasies; in essence the cross clamping time in patients with the ascending/hemi-arch approach is surprisingly long with 144 minutes versus data from the current literature quoting between 97 and 109 minutes for cross clamping (2); nonetheless cross clamping the aorta should not be common practice during acute Type A aortic dissection repair anymore as it causes maldistribution of flow in the false and true lumen during cardio-pulmonary bypass (CPB) with often detrimental effect on distal organ perfusion. In most aortic centres this practice was therefore abolished some 20 years ago. Other parameters such as hypothermic cardiac arrest time in both groups are similar to published experiences in IRAD and other expert groups (2-6); interestingly, Rylski et al. found a 22% mortality with hemi-arch replacement and 29% with aggressive total arch replacement (2). Similarly, IRAD (4) reported 13.1% mortality with hemi-arch replacement and 17.1% with total arch repair, both trending higher than reported by Yang et al. (1). The best contemporary results are published by Kim et al. (5) reflecting a 30-day mortality of 9.7% with the hemi-arch replacement versus 13.4% death rate with total arch replacement; their long-term survival rate is shown in Table 1 and is better with the less extensive hemi-arch procedure (log rank test P=0.013). Freedom from reoperation was however similar between both techniques (Table 1). These authors also reported postsurgical permanent neurological dysfunction more frequently seen after total arch placement with 22.7% versus 6.3% post hemi-arch procedure (5); thus, this problem was significantly less frequent the limited approach than with total arch repair, and similar to the incidence reported by Yang et al. regardless of the extent of surgery (1).
Full table
With a view on accuracy an important issue is the way the follow-up data were retrospectively retrieved. We understand that data were gathered from patients’ survey requests with a 75.4% response rate (missing around one quarter if index patients) despite additional access to and reliance on medical charts, the national death index database and telephone inquiries. To be properly analysed be Kaplan-Meier estimation the follow-up response rate should be above at least 80%. Finally, the length of follow-up in terms of mortality varied immensely at an average of 5.3 years; the hemi-arch group had a follow-up to 5 years in close to half of the patients while similar follow-up in the total arch group was available in approximately one third of patients. With regards to the surviving patients there is unfortunately no imaging information on anatomic changes of the aorta over time, on aneurysmal degeneration of the false lumen in the dissected aorta or at least on some parameter indicative of progression, which could be potentially influenced by the type of surgery.
In summary, what have we learnt and what is the new knowledge and what is already known in this conundrum? It is difficult to find new pieces of information besides those excellent surgical outcomes in the setting of an acute type A aortic dissection; is this result of operations performed by extremely skilful surgeons with a dedicated team behind them and is this also true particularly in the setting of a replacement of a dissected aortic arch in the middle of the night? The data may tend to encourage surgeons to be more enthusiastic to go ahead with a replacement of the aortic arch which may be more liberally than needed, and dilute the application of rigid selection criteria as previously advocated (2-4). A closer look at their own retrospective and partially incomplete set of data reveals the impression that they also somehow apply an inherent process of selection among their patients for either method, which again is not new as a strategy. Indeed, selection is one of the most important factors as total arch replacement is a far more complex operation than a replacement of the ascending aorta especially in the acute setting. A fairly well accepted observation that could be challenged by Yang and co-authors article (1) and insinuate that a more complex operation such as an aortic arch replacement in the acute setting should be encouraged because of similar long-term outcomes with the advantage of a lower incidence of reoperations at no extra expenses such as a higher early mortality or perioperative stroke rate as previously reported (2-6).
A slightly different and smart analysis could be to use their database to compare patients in whom the proximal tear could be fully resected/sealed by whatever extend of surgery was necessary to fulfil this goal with another group of patients that were left with some residual primary or residual re-entry tear in the aortic arch and as a result a perfused false lumen left under systolic pressure. Such a comparison would have probably shown that sealing of the main proximal primary tear is the prognostically relevant success formula of any surgical approach (regardless of its complexity).
What to take home from this article and its critical appraisal? First, the extent and complexity of surgery required may vary according to the individual setting and anatomy in type A dissection. Second, surgeons on an on-call emergency rotational service need both experience to make decisions to choose the safest approach to provide the best early survival benefit, and expertise in complex surgical interventions to use them if needed. Experienced Aortic surgeons can achieve excellent short and long-term results in complex aortic procedures in the setting of an acute aortic Type A dissection. But this is not the day to day reality in how most Type A aortic dissection patients are dealt with nowadays. In the hands of cardiac surgeon who are less experienced in complex aortic surgery a far more complex surgical approach is not essential to save the life of critically ill patient who has suffered an acute Type A aortic dissection. The reward is the best possible initial outcome with regards to survival and associated complications. Second stage elective surgery or late staged endovascular repair are established or emerging new options in the toolbox of an aortic centre where patients can be referred to at a later stage. Finally, it needs to be absolutely clear that the primary goal of any surgical intervention in the setting of an emergency operation for acute type A aortic dissection is resection/closure of the proximal tear which defines surgical success and survival of the patient. With those two goals in mind the element of adventure should kept to a minimum. It is fair enough to quote one of the pioneers in complex aortic surgery and remember his wise words from 20 years ago (3); Dr. Kazui knew already that extended total arch replacement for acute type A aortic dissection could be justified in properly selected patients. He also claimed 20 years ago that it is necessary to assess whether the extended aortic replacement for acute type A aortic dissection in fact improves long-term results. This data has still not been provided and the community should make an effort to create suitable platforms (such as prospective compulsory registries) to collect granular procedural and outcomes data for all complex aortic surgery.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflict of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yang B, Norton EL, Shih T, et al. Late outcomes of strategic arch resection in acute type A aortic dissection. J Thorac Cardiovasc Surg 2019;157:1313-21.e2. [Crossref] [PubMed]
- Rylski B, Beyersdorf F, Kari FA, et al. Acute type A aortic dissection extending beyond ascending aorta: Limited or extensive distal repair. J Thorac Cardiovasc Surg 2014;148:949-54; discussion 954. [Crossref] [PubMed]
- Kazui T, Washiyama N, Muhammad BA, et al. Extended total arch replacement for acute type a aortic dissection: experience with seventy patients. J Thorac Cardiovasc Surg 2000;119:558-65. [Crossref] [PubMed]
- Larsen M, Trimarchi S, Patel HJ, et al. Extended versus limited arch replacement in acute Type A aortic dissection. Eur J Cardiothorac Surg 2017;52:1104-10. [Crossref] [PubMed]
- Kim JB, Chung CH, Moon DH, et al. Total arch repair versus hemiarch repair in the management of acute DeBakey type I aortic dissection. Eur J Cardiothorac Surg 2011;40:881-7. [PubMed]
- Dib B, Seppelt PC, Arif R, et al. Extensive aortic surgery in acute aortic dissection type A on outcome - insights from 25 years single center experience. J Cardiothorac Surg 2019;14:187. [Crossref] [PubMed]


