Mechanistic impact of outdoor air pollution on asthma and allergic diseases
Allergic diseases and asthma still remain a critical public health, medical and economic problem, and are, in fact, among the major causes of illness and disability for all ages, particularly in Taiwan. Unfortunately, the trend of disease incidence is still on the rise and has caused significant economic burden to the general public. Particularly, data from a recent survey of 24,999 first-grade students in Taiwan revealed an alarmingly high prevalence of physician-diagnosed asthma, allergic rhinitis, and atopic eczema, with 13.0%, 33.7% and 29.8%, respectively (1). This is, indeed, a disturbing trend, considering also the fact that allergic diseases are often a chronic condition for life. The initial assessment of Taiwan’s National Health Insurance (NHI) expenditures for asthma* indicated that the annual healthcare cost is estimated to be more than 4 billion (NT$), when all outpatient visits and hospital admissions with asthma as one of the first three diagnoses were included*. In fact, the treatment and care for severe asthma represented more than half of the expenditure incurred. This significant increase in health cost is alarming, which highlights an urgent need for better understanding of the etiology and its causative mechanisms in order to improve treatment and develop effective prevention strategies.
Mechanistic aspects of air pollution’s effects
In the past decades, the increase in our understanding of the pathogenesis of allergic diseases has been substantial, and a Th2-dominant immune response leading to tissue inflammation and remodeling has long been considered as the underlying mechanism (2). Recently, new evidence has also been presented in support of the importance of innate immunity at the level of dendritic cell and epithelial cell response in disease expression (3,4), and additional types of T-cell responses [e.g., Th17 and innate lymphoid cells (iLCs)] also likely contribute to the disease progression (5,6). In the context of asthma, which is characterized by intermittent reversible airway obstruction, persistent pulmonary inflammation, fibrosis and enhanced airway hyperresponsiveness are considered to be the leading cause, particularly in severe cases (7,8). However, our current understanding of the molecular and genetic basis of asthma and allergic diseases is still incomplete; also, a significant portion of the adult asthma population appears to be “non-atopic”, but the etiology of this unique population remains to be defined. In this context, there has been a wealth of evidence linking exposure to both outdoor and indoor air pollution, especially airborne PMs, to adverse effects on the allergic diseases, where PM’s surface materials are likely to be the culprit and many immune regulatory cell types and pulmonary resident cells could very well be the targets. Notably, local and systemic oxidative stress has emerged as the likely common link between pulmonary exposure and immune regulatory effects, and as a converging point of their mechanistic impact (9,10).
Many reviews have discussed the potential mechanisms of each individual component associated with PMs; for example, the functional impact of air pollutant gases, such as ozone, NO2 and CO, has been extensively studied and several recent reviews (11,12) have provided a detailed account of their important roles and, thus, will not be the subject of this current review; rather, this review attempts to illustrate a series of mechanistic events as the result of exposure to PMs and their surface chemical and metal components to establish the exposure-disease mechanistic relationship. Specifically, the focus will be on the role of oxidative stress response that may explain the relationship between particulate air pollutants and allergic diseases; also, the recent documented importance of the aryl hydrocarbon receptor (AhR) with high binding affinity to environmental PAHs in immune regulation is discussed. While PMs are also known to contain biological components, including allergens and those from bacterial sources [e.g., lipopolysaccharide (LPS)], the impacts of those biological materials have been extensively reviewed and will not be the emphasis of this review.
PMs and their modulatory activities
PMs, a major component of air pollution, are generally derived from various anthropogenic, industrial activities and traffic-related sources primarily by coal and oil fuel combustion, while the combustion-derived particles can be originated from a number of sources, including diesel soot, welding fume, carbon black, coal or oil fly ash. In fact, human exposure to those particles has increased markedly over the past century; in particular, diesel exhaust has been estimated to account for up to 80% of human exposure. PMs typically contain a mixture of particles with different origins, size and composition, and are commonly grouped into the following categories: PM10 is defined as PM less than 10 mm, while those between 0.1 and 2.5 mm (PM2.5) are ‘fine’ in size and particles less than 0.1 mm are regarded as being ‘ultrafine’ (UF). It is worth noting that PM10 is often referred to those containing both fine and ultrafine particles (UFPs), and PM2.5 encompasses both fine and UFPs. World Health Organization (WHO) has set the daily exposure limits at 25 and 50 μg/m3, respectively, for PM2.5 and PM10, and an annual PM2.5 limit at less than 10 μg/m3 (13). At present, there are no recommendations made for daily or annual exposure limits for UFPs globally.
The potency of PMs in mediating the health impact is dependent, in part, on their deposition in the airways and the composition of their surface components (14). A variety of PMs are primarily composed of carbon cores and a host of organic chemicals and reactive metals on their surface, the composition of which may vary, depending on the source of the pollutant, the meteorological conditions, industrial activity and traffic density. PM10 are usually trapped in the upper airways, while PM2.5 particles, particularly the UFP fraction, can penetrate deep into the alveolar portions of the lung and those water-soluble components has been demonstrated, in fact, to be able to translocate directly through the alveolar capillaries into the circulation. Because of this size-dependent deposition, PM2.5 and UFPs, particularly diesel exhaust particles (DEPs) and residue oil fly ash (ROFA), have received much attention, since they possess, inherently, larger surface area for a given mass, and hence carrying larger amounts of reactive chemicals into the peripheral airways.
While the exact mechanisms of PM’s health effects remain to be fully defined, recent advancement in the study of various cellular and animal models has clearly documented the role of PMs in regulating many aspects of the respiratory functions, innate and adaptive immunity. In this regard, ROFA with chemically complex mixture of sulfates, carbon- and nitrogen-containing compounds and metals (primarily vanadium), has the capacity to influence immunity and induce injury in various experimental systems. Notably, several early studies have provided evidence that the composition of soluble metals and sulfate leached from ROFA is critical in the development of airway hyper-reactivity and lung injury (15). For example, in respiratory epithelial cells, ROFA mediated its impact on activation of signaling events and cytokine/chemokine production (16). Interestingly, redox-active vanadium, a major ROFA surface metal, was shown to be able to generate similar responses, whereas catalytically active iron and nickel compounds had no such effect. Conversely, treatment of the cells with deferoxamine, a metal chelating agent, reversed ROFA’s effects (17). In vivo, monkeys exposed to vanadium-containing compounds demonstrated neutrophilic inflammation in both the bronchi and the distal lung, which was accompanied by significant obstruction in the airways, supporting their importance in mediating ROFA’s effect (18). In addition, ROFA has been shown to enhance allergen-induced pulmonary allergic response in mouse models of asthma, with significant elevations in allergen-mediated eosinophilia and airway hyperresponsiveness (19-22). Again, this effect could be reproduced by the administration of ROFA’s metal leachate or its individual metallic constituents and could be abrogated by the addition of metal chelating agents (22,23). Interestingly, a very recent study (24) described a new mechanism of vanadate’s effect through augmenting the function of Toll-like receptor 4 (TLR4) by suppressing TLR4 degradation. These results suggest, therefore, that ROFA’s surface metals may be the key determinants in the induction and/or amplification of allergic responses.
Besides ROFA, DEPs are one of the most extensively studied PMs, which generally are within the PM2.5 category and account for most airborne PM exposure in major cities (25). Studies have shown that DEPs can modify the immune response and airway inflammatory processes in both animals and humans (26,27). The adjuvant activity of DEP is particularly noted in an elegant study of DEP’s effect on the development of IgE response to a “neo-antigen”, keyhole limpet hemocyanin (KLH), to which humans are not exposed (28). Diaz-Sanchez and her colleagues presented evidence showing that intranasal exposure of human subjects with KLH alone led to the generation of anti-KLH IgG and IgA, but not IgE, Abs. In contrast, when the study subjects were exposed to DEPs prior to the KLH challenge, the majority of the subjects developed anti-KLH—specific IgE Abs, while the levels of specific IgG and IgA Abs were similar to those with KLH sensitization alone. The elevated IgE levels were found to be associated with increased IL-4, but not IFN-γ, levels in the nasal lavage fluid. These studies demonstrate that DEPs can act as mucosal adjuvant in enhancing the IgE response to allergens and amplifying the allergic responses. Further, the finding of DEP’s adjuvant activity was corroborated from the studies of allergen-induced responses, where DEP challenge significantly enhanced nasal ragweed-specific IgE and skewed cytokine production to a Th2 pattern in humans (29). Further, DEP’s enhancing effect has recently been broadened to include its prenatal influence on allergic sensitization. Interestingly, the risk was found to be particularly evident in subjects carrying homozygous variant genotype of the gene encoding glutathione-S-transferase M1 (GSTM1) (30). The fact that aeroallergens can also be found to associate with PMs highlights the importance of PM exposure in contributing to the enhanced allergic response (31).
Moreover, it has recently been demonstrated that co-exposure to DEP and HDM significantly enhanced airway hyperresponsiveness as compared with HDM exposure alone, concomitant with a mixed Th2 and Th17 response, including IL-13/IL-17A double-producing T cells (32). Neutralization of IL-17A prevented DEP-induced airway hyperresponsiveness. Significantly, high DEP-exposed children with allergic asthma, 32.2% of them had more frequent asthma symptoms and higher serum IL-17A levels as opposed to low DEP-exposed children, suggesting an expansion of Th17 cells in DEP-exposed subjects with severe asthma. In addition, PM has recently been shown to suppress regulatory T cells (Treg) through its ability in causing hypermethylation of the gene encoding the Treg-transcription factor, Foxp3, in the peripheral blood of children with asthma (33). The reduction in Treg population upon exposure to PMs could be important in potentiating the allergic response, although further studies are needed to confirm this finding. It is also of interest to note that acute exposure of infant mice to a chemically defined environmentally persistent free radicals (EPFR) with an organic pollutant 1,2-dichlorobenzene (DCB-230) led to epithelial-to-mesenchymal transition (EMT) in the lungs, as evidenced by lineage tracing studies and by the expression of both epithelial E-cadherin and mesenchymal α-smooth muscle actin (α-SMA) proteins (34).
In a direct intervention-type of the study, exposure of healthy human subjects to DEP (300 μg/m3 for 1 hour) in a chamber caused an increase in inflammatory cells (neutrophils, B lymphocytes, mast cells, CD4+ and CD8+ T lymphocytes) along with up-regulation of ICAM-1 and vascular cell adhesion molecule-1 in the lungs (35). However, when atopic asthmatics were exposed to the same amount of DEP, no significant changes in the levels of neutrophils, eosinophils and IL-8 expression were noted, due, perhaps in part, to the pre-existing inflammatory response (36). Further, exposure to DEP (PM10, 300 µg/m3) enhanced the expression of EGFR and its phosphorylation at the tyrosine residue (Tyr 1173), concomitant with increased activation of the JNK, AP-1, p38 MAPK and NF-kB pathways as well as cytokine production in the airway mucosa of healthy subjects (37,38).
While these results are informative, the mechanisms through which exposure to ROFA or DEP mediates the Th2, Th17, IgE and epithelial responses remain unclear. It is also, at present, unclear as to the exact components responsible for the observed effects mediated by PMs or DEP. In general, DEPs consist of polycyclic aromatic hydrocarbons (PAHs) and transition metals, and as in ROFA, DEP-bound transition metals may play a role in generating oxidative stress and inflammatory responses. Further, their hydrophobic nature allows them to diffuse through cell membranes, and PAHs may interact with a unique cellular chemical sensor, aryl hydrocarbon receptor (AhR; see below), thereby activating the cellular response. In fact, recent discoveries regarding AhR and environmental toxicant interaction and its influence on immune responses have highlighted the potential importance of AhR in linking the environmental exposure and the development of asthma and allergic diseases.
Involvement of AhR signaling pathways in controlling cellular homeostasis
AhR is a ligand-activated transcription factor from the Per-Arnt-Sim (PAS) superfamily and has been shown to be involved in maintaining cellular homeostasis [for recent review, see (39,40)]. Originally discovered as a receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), AhR has also been recognized as a receptor for many of the common environmental contaminants, including polychlorinated biphenyls (PCBs) and PAHs, such as benzo[α]pyrene (BaP). Upon ligand interaction, AhR in the cytoplasm translocates into the nucleus and binds to the specific regulatory DNA sequences known as dioxin response elements (DREs) located within the promoters of target genes. Those target genes include those involving detoxification enzymes and activities, e.g., CYP1A1 and CYP1B1 etc., as well as those known to be critical in immune regulation, including inflammatory (TNF-α and IL-6) and T-cell differentiation genes (Foxp3, IL-17 and GATA3) (41). Also, beside the direct effect on target gene transcription, AhR may also influence the activation of key signaling events involving NFkB, MAPK, STAT1 and STAT5 that have been observed in many experimental systems (42). In particular, recent discoveries of AhR and its influence on the balance of Tregs and Th17 cells (43,44), and its impact on γδ+ T (45), intraepithelial lymphocytes (46), lymphoid follicles (47), as well as dendritic cell function (48) highlight the potential importance of the AhR-ligand axis in the expression of allergic and inflammatory diseases. We have recently discovered that in stimulated mast cells, a critical cell type in the regulation of allergic responses and mucosal immunity (41), exposure to environmental chemicals, such as dioxin and BaP, resulted in enhanced mast cell signaling, degranulation, mediator and cytokine release, as well as the in vivo anaphylactic response (49). Interestingly, known endogenous ligands, including the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole (FICZ) and a key metabolite of tryptophan, kynurenine, were able to mediate similar effects (49,50). As a corollary, two recent studies (51,52) supported the importance of the AhR ligands in regulating the mast cell response. Therefore, it is logical to speculate that the AhR pathway may serve as a critical bridge linking environmental exposure and allergic diseases. In support of this, experimental evidence has suggested that TCDD, the classical AhR ligand, was able to enhance IgE production and aggravate allergic diseases (53). Also, previous studies have pointed to a possible effect of the exposure to PAHs and allergic diseases (54).
Moreover, the phenotypic alterations found in AhR-null mouse models have suggested the endogenous function of AhR, independent of xenobiotic metabolism, in controlling cellular homeostasis (47,49). This was especially noted in our recent finding of significant mast cell deficiency in AhR-null mice, due to defective calcium signaling and mitochondrial function. Consequently, AhR-null mast cells responded poorly to stimulation, demonstrating a critical role of AhR signaling in maintaining mast cell homeostasis (49). These results suggest that exposure to environmental pollutants may modify the homeostatic state through their ability to influence the immune response directly or indirectly through the disruption of the normal endogenous function of AhR, and together may contribute to the pathogenesis and symptoms of allergic diseases. In this context, AhR is known to be a chemical sensor initiating the detoxification process through its ability to induce both phase I and II enzymes. Disruption of this critical function by competitive exogenous ligands or by mechanisms causing fluctuation in its expression level would, therefore, have a direct impact on the normal functioning of the detoxification process. It is, perhaps, the dysregulated detoxification, or increased metabolites thereof, in enhancing the cellular response and/or causing the inflammatory responses following the environmental insult. Further investigations into these interesting possibilities may provide additional insights in our understanding of the exposure-disease relationship.
Oxidative stress response and its functional consequences
As discussed above, one of the most consistent PM-mediated immunoregulatory and respiratory effects involves PM-driven oxidative stress (13), a feature likely to be mediated by those PMs referred to as “EPFRs”, in which PAHs are chemisorbed to the surface of PM through transition metal oxides. Indeed, the role of oxidative stress in allergic diseases is gaining increasing scientific attention. Exposure to a variety of pollutants has been shown to be able to generate a collection of oxygen-derived free radicals and oxidants, such as hydrogen peroxide and peroxynitrite. For example, combustion-derived PMs are known to be highly oxidizing and are capable of generating free radicals, through, in part, their surface metals involved in redox cycling or the depletion of anti-oxidant glutathione and protein-bound sulfhydryl groups (55,56). It has been postulated that transition metals found in ROFA, particularly vanadium, may generate ROS in an Fenton-like chemical reaction, leading to the subsequent activation of cellular signaling, transcription and induction of inflammatory mediator release and airway hyperresponsiveness [for detailed review see (10) and the references therein].
Oxidative stress occurs not only as a result of environmental exposure to air pollution but also from inflammation, thus perpetuating the oxidative stress response (57,58). Various components of air pollution, including gases and PMs, are known to be able to induce oxidative stress either by direct induction of reactive oxygen species (ROS) or through the secondary induction of local inflammatory processes which lead to the secondary production of ROS (59). Other components of air pollution that may contribute to oxidant generation include transition metals, such as chromium, iron, manganese, vanadium and copper. While the detailed mechanisms remain to be elucidated, oxidative stress has been shown to mediate multiple effects and is associated with activation of inflammatory cells and release of pro-inflammatory cytokines and mediators (60,61). One of the possible earlier events may be as the consequence of the cellular detoxification itself in the endoplasmic reticulum (ER), particularly with regard to the detoxification process of PAH exposure. ER is critical for protein folding and secretion, calcium homeostasis, lipid biosynthesis and detoxification of xenobiotic substances. Any disturbance in the redox balance within the ER may lead to the accumulation of unfolded proteins in the ER lumen, a condition referred to as “ER stress”. In response to ER stress, an adaptive signaling pathway, known as the unfolded protein response (UPR), is activated, which intersects with many different inflammatory and stress signaling pathways (62,63).
ER stress has been implicated in many inflammatory diseases (64,65), although the roles of ER stress in the pathogenesis of allergic inflammation remain to be elucidated. A recent study (66) of PM2.5 exposure and ER stress response may shed some new lights on this possibility. The study by Laing et al. provided evidence that exposure of epithelial cells to PM2.5 differentially activated the UPR branches, leading to ER stress-induced apoptosis. Also, we have found that exposure of mast cells to AhR ligands resulted in increased expression of several ER stress response-associated markers (unpublished observation). Moreover, AhR ligands have been shown to increase ROS production, perhaps as the consequence of the increased expression of AhR’s target genes, CYP1A1 (49,67) and a member of the membrane NADPH oxidase complex, p40phox (68). Therefore, chronic exposure to PM2.5 or its derivatives may interfere with the normal functioning of the ER and stimulate the ER stress response via, in part, AhR, leading to dysregulated allergic response.
Mechanistically, increased oxidative stress is known to mediate the activation of various signal transduction pathways and transcription factors, including MAPK kinases, phosphoinositol-3 kinase, NF-kB and AP-1 (69), all of which are known to be crucially involved in cellular activation and inflammation (70). Further, the increase in the levels of ROS can also cause oxidative modifications of lipids and proteins, thereby altering their functions. For example, the loss of superoxide dismutase (SOD; an important anti-oxidant defense enzyme) activity by oxidative modification has been shown in asthmatic airway epithelial cells (71), and has been evaluated as a surrogate marker of oxidant stress (57). Further, our recent study has shown that an important non-receptor tyrosine phosphatase, SHP-2, is a target of oxidation via an AhR- and ROS-dependent mechanism, leading to reduced activity of its phosphatase activity, and hence upregulating cellular activation in mast cells (49). Similarly, several studies have demonstrated that many key enzymes involved in cellular metabolism, including glycolysis, are targets of tyrosine nitration or S-nitrosylation (72), thereby reducing the activity or function of the parent proteins.
Moreover, increased oxidative stress response may lead to enhanced peroxidation of polyunsaturated fatty acids (PUFAs) and their metabolites. More than 20 lipoperoxidation end-products have been identified (73), including malondialdehyde (MDA), 4-hydroxyalkenals and isoprostanes (74). Importantly, MDA and 4-HNE are known to react with a variety of proteins, lipids and nucleic acids, and they are thought to have pro-inflammatory effect and contribute to the pathogenesis of human chronic diseases (75-77). An additional consequence of upregulated oxidative stress response involves the generation and liberation of lipid mediators, including prostaglandins, leukotrienes and lipoxins, in a tightly regulated, coordinated, cell- and context-specific manner (78). Lipid mediators are known to be important as initiators of pro- and anti-inflammatory responses (79,80). Recently, we found that AhR ligands could significantly up-regulate LTC4 and LTB4 production in mast cells, and that AhR signaling-induced ROS and AhR ligand’s enhancing effect could be inhibited, at least in part, by inhibitors for 5-lipoxygenase (5-LO) and COX1, but not COX2, suggesting the possible functional role of eicosanoid metabolites in amplifying the effects of AhR ligands, for example, PAHs.
Conclusions and future challenges
Current epidemiological evidence from the study of various populations supports a link between air pollution and the development and exacerbation of asthma and allergic diseases. Also, recent advancement in the knowledge about the effect of PM exposure clearly indicates its diverse impact on many different cell types at different levels of immune regulation. One intriguing and plausible hypothesis is that the expression and the increased prevalence of allergic diseases could be attributable directly to those environmental pollutants through their ability to increase the oxidant burden and inflammatory response or their immune “adjuvant” activities, and together contribute to the pathogenesis of asthma and allergic diseases. Thus, how tissue resident cells and immune regulatory cell types maintain the homeodynamic redox balance in response to environmental insult may represent an important mechanism in the genesis of, and/or the progression in, asthma and allergic diseases. Based on accumulated knowledge, Figure 1 illustrates, schematically, a likely sequential event initiated following the exposure to environmental pollutants, leading to the expression of asthma.
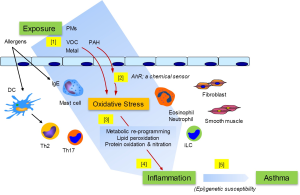
While current assessment of air pollution’s effect has been, understandably, limited to the evaluation of individual component, the highly dynamic “multi-pollutants effects” (81,82), which are expected to have additive or synergistic effects, will need to be examined in order to fully appreciate the extent to which the air pollution exerts its impact. Future effort will also be needed to use cutting-edge technology and analytical tools to dissect the complexity of air pollution and its interaction with other environmental risk factors, such as life style and eating habits, integrating the concept of “exposome” as originally proposed by Wild (83) to encompass life-course environmental exposures (including lifestyle factors) for better and more complete environmental exposure data for etiologic research (84). Mechanistically, additional studies will be needed to define the mechanisms by which air pollution (particularly PMs)-mediated oxidative stress impacts the expression of allergic diseases. Future efforts will also require “-omics” approaches, including metabolomics, to identify the disease relevant protein and lipid adducts and/or metabolites, as well as their functional consequences. Also, it has long been appreciated that PMs may trigger the alterations in autonomic nervous system leading to aberrant pulmonary function. Therefore, future effort in elucidating the responsible particle constituents and their underlying mechanisms will be needed.
The issue of genetic susceptibility to air pollution specifically and to the environment as a whole is another major challenge facing this field of investigation. So far, there has been no “genetic variant(s)” that can unequivocally define asthma and allergic diseases, and there have been no genetic markers that are generally applicable in different ethnic backgrounds (85). Genetic heterogeneity and ethnical difference may all contribute to the failure to discover the bona fide “asthma gene(s)”. More importantly, the issue of gene-environment interaction has not been properly addressed and has only been included in a relatively few genetic studies. Therefore, a critical challenge in the future is to investigate whether and how variations in the human genome (polymorphisms) are able to modify the effect of exposures to environmental pollutants and chemicals or vice versa. It has been noted that the endogenous activity of the antioxidant mechanisms varies greatly between individuals, and that the ability to manage oxidative stress is altered in the lungs of asthmatics (86), leading to excessive ROS and its subsequent effects. In the future, the pursuit of this important area of research would ideally incorporate the environmental-wide association studies (EWAS, or exposome-wide association studies), which has been fruitful in different disease context (87,88). It is relevant to note that in evaluating the features of the susceptible population to PM-related health effects, Sacks et al. (89) recently reported a group of characteristics, including life stage (i.e., children and older adults), preexisting cardiovascular or respiratory diseases, genetic polymorphisms and low-socioeconomic status, contributing to the increased risk of PM-related health effects.
In parallel, additional analyses utilizing powerful tools will be required for discovering and developing the next-generation of biomarkers to monitor the response to exposure, to capture the earlier pathologic events of inflammation and oxidative response as the consequence of exposure to environmental pollutants and chemicals, and to predict future clinically relevant events. For example, the fraction of exhaled NO (FeNO) (90,91), which has been used to distinguish asthma from non-asthma; but, it is a relatively non-specific marker of response and has, thus far, no value in defining a more specific disease phenotype and its severity. Also, the generation of lipid peroxidation products, including isoprostanoids and 4-HNE, as the result of the oxidative stress response has been evaluated (92,93). However, a major limitation with most of these parameters is their poor specificity and reproducibility when evaluating the disease progression and severity.
Recently, the developmental origin of human diseases has been an active field of investigation, wherein emerging evidence has suggested that gene-environmental interactions during pregnancy enhance the disease susceptibility through, in part, epigenetic mechanisms. This has been best studied in the context of cardiovascular and metabolic diseases, and the epidemic rise in chronic diseases, including allergic diseases, also highlights the likelihood of their maternal origin. These collective efforts will be critical in identifying important environmental risks, the time window of exposure and in defining the molecular mechanisms underlying the exposure-disease relationship. This should lead to better understanding of the role of environmental risk factors, including air pollution, in asthma and allergic diseases and ultimately to the development of better prevention strategies. Further, the outcomes of this collective effort are expected to provide crucial information for developing evidence-based interventional approaches and regulatory guidelines. Importantly, the success of this type of integrated approaches will help in improving public health awareness and ultimately the health of the general population, with a concomitant significant reduction in economic burden.
Footnote: *Initial assessment of the asthma burden in 2012 by Dr. Li-Kuan Chen, National Health Research Institutes, as a part of the “Consortium for Taiwan Asthma Study (CTAS)”.
Acknowledgements
SKH is supported in part by grants from National Health Research Institutes, Taiwan, NHRI-102A1-PDCO-03010201 and Ministry of Health, Taiwan (EODOH01). KFC is supported by a British Heart Foundation grant examining the effect of diesel exhaust particles in London and by NIHR Respiratory Biomedical Research Unit at the Royal Brompton NHS Foundation Trust and Imperial College London. He is also a Senior Investigator of NIHR UK and a Visiting Professor of Guangzhou Medical College.
Disclosure: The authors declare no conflict of interest.
References
- Wu WF, Wan KS, Wang SJ, et al. Prevalence, severity, and time Trends of allergic conditions in 6-to-7-year-old schoolchildren in Taipei. J Investig Allergol Clin Immunol 2011;21:556-62. [PubMed]
- Barnes PJ. Pathophysiology of allergic inflammation. Immunol Rev 2011;242:31-50. [PubMed]
- Lambrecht BN, Hammad H. The role of dendritic and epithelial cells as master regulators of allergic airway inflammation. Lancet 2010;376:835-43. [PubMed]
- Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol 2010;10:838-48. [PubMed]
- Wisniewski JA, Borish L. Novel cytokines and cytokine-producing T cells in allergic disorders. Allergy Asthma Proc 2011;32:83-94. [PubMed]
- Licona-Limón P, Kim LK, Palm NW, et al. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14:536-42. [PubMed]
- Murphy DM, O’Byrne PM. Recent advances in the pathophysiology of asthma. Chest. 2010;137:1417-26. [PubMed]
- Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2014. Available online: http://www.ginasthma.org/
- Nadeem A, Masood A, Siddiqui N. Oxidant--antioxidant imbalance in asthma: scientific evidence, epidemiological data and possible therapeutic options. Ther Adv Respir Dis 2008;2:215-35. [PubMed]
- Comhair SA, Erzurum SC. Redox control of asthma: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal 2010;12:93-124. [PubMed]
- Kim KH, Jahan SA, Kabir E. A review on human health perspective of air pollution with respect to allergies and asthma. Environ Int 2013;59:41-52. [PubMed]
- Jang AS. Particulate air pollutants and respiratory diseases. In: Haryanto B. eds. Air Pollution-A Comprehensive Perspective. Publisher: InTech, 2012:153-74.
- Saravia J, Lee GI, Lomnicki S, et al. Particulate matter containing environmentally persistent free radicals and adverse infant respiratory health effects: a review. J Biochem Mol Toxicol 2013;27:56-68. [PubMed]
- Stanek LW, Brown JS, Stanek J, et al. Air pollution toxicology--a brief review of the role of the science in shaping the current understanding of air pollution health risks. Toxicol Sci 2011;120 Suppl 1:S8-27. [PubMed]
- Ghio AJ, Silbajoris R, Carson JL, et al. Biologic effects of oil fly ash. Environ Health Perspect. 2002;110 Suppl 1:89-94. [PubMed]
- Samet JM, Stonehuerner J, Reed W, et al. Disruption of protein tyrosine phosphate homeostasis in bronchial epithelial cells exposed to oil fly ash. Am J Physiol 1997;272:L426-32. [PubMed]
- Carter JD, Ghio AJ, Samet JM, et al. Cytokine production by human airway epithelial cells after exposure to an air pollution particle is metal-dependent. Toxicol Appl Pharmacol 1997;146:180-8. [PubMed]
- Knecht EA, Moorman WJ, Clark JC, et al. Pulmonary effects of acute vanadium pentoxide inhalation in monkeys. Am Rev Respir Dis 1985;132:1181-5. [PubMed]
- Gavett SH, Madison SL, Stevens MA, et al. Residual oil fly ash amplifies allergic cytokines, airway responsiveness, and inflammation in mice. Am J Respir Crit Care Med 1999;160:1897-904. [PubMed]
- Lambert AL, Dong W, Winsett DW, et al. Residual oil fly ash exposure enhances allergic sensitization to house dust mite. Toxicol Appl Pharmacol 1999;158:269-77. [PubMed]
- Lambert AL, Dong W, Selgrade MK, et al. Enhanced allergic sensitization by residual oil fly ash particles is mediated by soluble metal constituents. Toxicol Appl Pharmacol 2000;165:84-93. [PubMed]
- Arantes-Costa FM, Lopes FD, Toledo AC, et al. Effects of residual oil fly ash (ROFA) in mice with chronic allergic pulmonary inflammation. Toxicol Pathol 2008;36:680-6. [PubMed]
- Knecht EA, Moorman WJ, Clark JC, et al. Pulmonary reactivity to vanadium pentoxide following subchronic inhalation exposure in a non-human primate animal model. J Appl Toxicol 1992;12:427-34. [PubMed]
- Zelnikar M, Benčina M, Jerala R, et al. Vanadate from air pollutant Inhibits Hrs-dependent endosome fusion and augments responsiveness to Toll-like receptors. PLoS ONE 2014;9:e99287. [PubMed]
- Riedl M, Diaz-Sanchez D. Biology of diesel exhaust effects on respiratory function. J Allergy Clin Immunol 2005;115:221-8. [PubMed]
- Provoost S, Maes T, Willart MA, et al. Diesel exhaust particles stimulate adaptive immunity by acting on pulmonary dendritic cells. J Immunol 2010;184:426-32. [PubMed]
- Deiuliis JA, Kampfrath T, Zhong J, et al. Pulmonary T cell activation in response to chronic particulate air pollution. Am J Physiol Lung Cell Mol Physiol 2012;302:L399-409. [PubMed]
- Diaz-Sanchez D, Garcia MP, Wang M, et al. Nasal challenge with diesel exhaust particles can induce sensitization to a neoallergen in the human mucosa. J Allergy Clin Immunol 1999;104:1183-8. [PubMed]
- Diaz-Sanchez D, Tsien A, Fleming J, et al. Combined diesel exhaust particulate and ragweed allergen challenge markedly enhances human in vivo nasal ragweedspecific IgE and skews cytokine production to a T helper cell 2-type pattern. J Immunol 1997;158:2406-13. [PubMed]
- Perzanowski MS, Chew GL, Divjan A, et al. Early-life cockroach allergen and polycyclic aromatic hydrocarbon exposures predict cockroach sensitization among inner-city children. J Allergy Clin Immunol 2013;131:886-93. [PubMed]
- Zhou J, Ito K, Lall R, et al. Time-series analysis of mortality effects of fine particulate matter components in Detroit and Seattle. Environ Health Perspect 2011;119:461-6. [PubMed]
- Brandt EB, Kovacic MB, Lee GB, et al. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol 2013;132:1194-204. [PubMed]
- Nadeau K, McDonald-Hyman C, Noth EM, et al. Ambient air pollution impairs regulatory T-cell function in asthma. J Allergy Clin Immunol 2010;126:845-52. [PubMed]
- Thevenot PT, Saravia J, Jin N, et al. Radical-containing ultrafine particulate matter initiates epithelial-to-mesenchymal transitions in airway epithelial cells. Am J Respir Cell Mol Biol 2013;48:188-97. [PubMed]
- Salvi S, Blomberg A, Rudell B, et al. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med 1999;159:702-9. [PubMed]
- Stenfors N, Nordenhall C, Salvi SS, et al. Different airway inflammatory responses in asthmatic and healthy humans exposed to diesel. Eur Respir J 2004;23:82-6. [PubMed]
- Pourazar J, Mudway IS, Samet JM, et al. Diesel exhaust activates redox-sensitive transcription factors and kinases in human airways. Am J Physiol Lung Cell Mol Physiol 2005;289:L724-30. [PubMed]
- Pourazar J, Blomberg A, Kelly FJ, et al. Diesel exhaust increases EGFR and phosphorylated C-terminal Tyr 1173 in the bronchial epithelium. Particle and Fibre Toxicology 2008;5:8. [PubMed]
- Hao N, Whitelaw ML. The emerging roles of AhR in physiology and immunity. Biochem Pharmacol 2013;86:561-70. [PubMed]
- Nguyen NT, Hanieh H, Nakahama T, et al. The roles of aryl hydrocarbon receptor in immune responses. Int Immunol 2013;25:335-43. [PubMed]
- Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol 2008;8:478-86. [PubMed]
- Veldhoen M, Duarte JH. The aryl hydrocarbon receptor: fine-tuning the immune-response. Curr Opin Immunol 2010;22:747-52. [PubMed]
- Veldhoen M, Hirota K, Westendorf AM, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 2008;453:106-9. [PubMed]
- Quintana FJ, Basso AS, Iglesias AH, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 2008;453:65-71. [PubMed]
- Kadow S, Jux B, Zahner SP, et al. Aryl hydrocarbon receptor is critical for homeostasis of invariant gamma delta T cells in the murine epidermis. J Immunol 2011;187:3104-10. [PubMed]
- Li Y, Innocentin S, Withers DR, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 2011;147:629-40. [PubMed]
- Kiss EA, Vonarbourg C, Kopfmann S, et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 2011;334:1561-5. [PubMed]
- Nguyen NT, Kimura A, Nakahama T, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci USA 2010;107:19961-6. [PubMed]
- Zhou Y, Tung HY, Tsai YM, et al. Aryl hydrocarbon receptor controls murine mast cell homeostasis. Blood 2013;121:3195-204. [PubMed]
- Kawasaki H, Chang HW, Tseng HC, et al. A tryptophan metabolite, kynurenine, promotes mast cell activation through aryl hydrocarbon receptor. Allergy 2014;69:445-52. [PubMed]
- Maaetoft-Udsen K, Shimoda LM, Frokiaer H, et al. Aryl hydrocarbon receptor ligand effects in RBL2H3 cells. J Immunotoxicol 2012;9:327-37. [PubMed]
- Sibilano R, Frossi B, Calvaruso M, et al. The Aryl Hydrocarbon Receptor Modulates Acute and Late Mast Cell Responses. J Immunol 2012;189:120-7. [PubMed]
- Kimata H. 2,3,7,8-tetrachlorodibenzo-p-dioxin selectively enhances spontaneous IgE production in B cells from atopic patients. Int J Hyg Environ Health 2003;206:601-4. [PubMed]
- Lubitz S, Schober W, Pusch G, et al. Polycyclic aromatic hydrocarbons from diesel emissions exert proallergic effects in birch pollen allergic individuals through enhanced mediator release from basophils. Environ Toxicol 2010;25:188-97. [PubMed]
- Stohs SJ, Bagci D, Hassoun E, et al. Oxidative mechanisms in the toxicity of chromium and cadmium ions. J Environ Pathol Toxicol Oncol 2001;20:77-88. [PubMed]
- Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem 2005;12:1161-208. [PubMed]
- Bowler RP. Oxidative stress in the pathogenesis of asthma. Curr Allergy Asthma Rep 2004;4:116-22. [PubMed]
- Li YJ, Takizawa H, Kawada T. Role of oxidative stresses induced by diesel exhaust particles in airway inflammation, allergy and asthma: their potential as a target of chemoprevention. Inflamm Allergy Drug Targets 2010;9:300-5. [PubMed]
- Ghio AJ, Carraway MS, Madden MC. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J toxicol envir health Part B Critical reviews 2012;15:1-21. [PubMed]
- Paredi P, Kharitonov SA, Barnes PJ. Analysis of expired air for oxidation products. Am J Respir Crit Care Med 2002;166:S31-7. [PubMed]
- Wood LG, Gibson PG, Garg ML. Biomarkers of lipid peroxidation, airway inflammation and asthma. Eur Respir J 2003;21:177-86. [PubMed]
- Hasnain SZ, Lourie R, Das I, et al. The interplay between endoplasmic reticulum stress and inflammation. Immunol Cell Biol 2012;90:260-70. [PubMed]
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 2011;334:1081-6. [PubMed]
- Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol 2008;8:663-74. [PubMed]
- Bhandary B, Marahatta A, Kim HR, et al. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int J Mol Sci 2012;14:434-56. [PubMed]
- Laing S, Wang G, Briazova T, et al. Airborne particulate matter selectively activates endoplasmic reticulum stress response in the lung and liver tissues. Am J Physiol Cell Physiol 2010;299:C736-49. [PubMed]
- Kopf PG, Walker MK. 2,3,7,8-tetrachlorodibenzo-p-dioxin increases reactive oxygen species production in human endothelial cells via induction of cytochrome P4501A1. Toxicol Appl Pharmacol 2010;245:91-9. [PubMed]
- Wada T, Sunaga H, Ohkawara R, et al. Aryl Hydrocarbon Receptor Modulates NADPH Oxidase Activity via Direct Transcriptional Regulation of p40phox Expression. Mol Pharmacol 2013;83:1133-40. [PubMed]
- Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J 2006;28:219-42. [PubMed]
- Carvalho H, Evelson P, Sigaud S, et al. Mitogen-activated protein kinases modulate H(2)O(2)-induced apoptosis in primary rat alveolar epithelial cells. J Cell Biochem 2004;92:502-13. [PubMed]
- Malik AI, Storey KB. Transcriptional regulation of antioxidant enzymes by FoxO1 under dehydration stress. Gene 2011;485:114-9. [PubMed]
- Ischiropoulos H. Protein tyrosine nitration—An update. Archives of Biochemistry and Biophysics 2009;484:117-21. [PubMed]
- Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med 2009;47:469-84. [PubMed]
- Leopold JA, Loscalzo L. Oxidative Risk for Atherothrombotic Cardiovascular Disease. Free Radic Biol Med 2009;47:1673-706. [PubMed]
- Spickett CM. The lipid peroxidation product 4-hydroxy-2-nonenal: Advances in chemistry and analysis. Redox Biol 2013;1:145-52. [PubMed]
- Riahi Y, Cohen G, Shamni O, et al. Signaling and cytotoxic functions of 4-hydroxyalkenals. Am J Physiol Endocrinol Metab 2010;299:E879-86. [PubMed]
- Cohen G, Riahi Y, Sunda V, et al. Signaling properties of 4-hydroxyalkenals formed by lipid peroxidation in diabetes. Free Radic Biol Med 2013;65:978-87. [PubMed]
- Boyce JA. Eicosanoids in asthma, allergic inflammation, and host defense. Curr Mol Med 2008;8:335-49. [PubMed]
- Sadik CD, Luster AD. Lipid-cytokine-chemokine cascades orchestrate leukocyte recruitment in inflammation. J Leukoc Biol 2012;91:207-15. [PubMed]
- Ohnishi H, Miyahara N, Gelfand EW. The role of leukotriene B(4) in allergic diseases. Allergol Int 2008;57:291-8. [PubMed]
- Johns DO, Stanek LW, Walker K, et al. Practical advancement of multipollutant scientific and risk assessment approaches for ambient air pollution. Environ Health Perspect 2012;120:1238-42. [PubMed]
- Billionnet C, Sherrill D, Annesi-Maesano I. GERIE study. Estimating the health effects of exposure to multi-pollutant mixture. Ann Epidemiol 2012;22:126-41. [PubMed]
- Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev 2005;14:1847-50. [PubMed]
- Rappaport SM. Implications of the exposome for exposure science. J Expo Sci Environ Epidemiol 2011;21:5-9. [PubMed]
- Akhabir L, Sandford AJ. Genome-wide association studies for discovery of genes involved in asthma. Respirology 2011;16:396-406. [PubMed]
- Dworski R. Oxidant stress in asthma. Thorax 2000;55:S51-3. [PubMed]
- Patel CJ, Bhattacharya J, Butte AJ. An environment-wide association study (EWAS) on type 2 diabetes mellitus. PLoS One 2010;5:e10746. [PubMed]
- Patel CJ, Chen R, Kodama K, et al. Systematic identification of interaction effects between genome- and environment-wide associations in type 2 diabetes mellitus. Hum Genet 2013;132:495-508. [PubMed]
- Sacks JD, Stanek LW, Luben TJ, et al. Particulate matter-induced health effects: who is susceptible? Environ Health Perspect 2011;119:446-54. [PubMed]
- Majid H, Kao C. Utility of exhaled nitric oxide in the diagnosis and management of asthma. Curr Opin Pulm Med 2010;16:42-7. [PubMed]
- Kostikas K, Minas M, Papaioannou AI, et al. Exhaled nitric oxide in asthma in adults: the end is the beginning? Curr Med Chem 2011;18:1423-31. [PubMed]
- Louhelainen N, Myllärniemi M, Rahman I, et al. Airway biomarkers of the oxidant burden in asthma and chronic obstructive pulmonary disease: current and future perspectives. Int J Chron Obstruct Pulmon Dis 2008;3:585-603. [PubMed]
- Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med 2001;163:1693-722. [PubMed]


