The changes of vaccinia related kinase 1 in grafted heart after rat heart transplantation
Introduction
Since the first successful heart transplantation by Christian Barnard in 1967, there have been over 60,000 heart transplants performed worldwide (1). Cardiac transplantation is a significantly used therapy for end-stage heart failure. Allogeneic graft survival is affected by acute chronic and rejection due to incomplete histocompatibility (2,3). The main reasons of allograft failure after heart transplantation are intractable acute rejection, primary graft dysfunction, and coronary graft disease (4). Improved cardiac function and myocardial cell survival after heart transplantation are significant to allograft. Many recent studies aimed to prolong allograft survival, thus improve the effectiveness of the heart transplantation (5).
The vaccinia-related kinases (VRKs) branched off early from the family of casein kinase (CK) I and compose a relatively uncharacterized family of the kinome (6). The VRKs were discovered due to their close sequence relation to the vaccinia virus B1R serine/threonine kinase (6). B1R is an early protein essential for viral replication (7). There are three members in VRKs family. However, these three kinases differ significantly in their regulatory domains, which are located at the C-terminus in the case of VRK1 and VRK2, and at the N-terminus in VRK3 (8).
It is well known that several Ser/Thr kinases called cyclin-dependent kinases (CDKs) play an important role in cell regulation by reversibly phosphorylating key molecules controlling cell cycle progression (9). Several cell protection mechanisms are based on the ability of the p53 protein to regulate the progression of the cell cycle, the induction of apoptosis, or replicative senescence (7). VRK1 is highly expressed in tumor cells with high proliferation rates (8). It has reported that VRK1 as the first step in a new pathway regulating p53 activity during cell proliferation and anti-apoptosis (7).
All data showed that VRK1 can regulate cell proliferation in many tumors and resist cell apoptosis. Thus, we examined whether cardiomyocytes of heterotopic heart transplantation express VRK1, VRK1 regulates the apoptosis of cardiomyocytes in our model. These evidences indicate that the VRK1 expression has increased after heart transplantation in allograft and isograft, and VRK1 may play a significant role of myocardial apoptosis after heterotopic heart transplantation in rats.
Materials and methods
Animals and heterotopic heart transplantation
Wistar (n=20) and Lewis rats (n=60) with an average body weight of 280 g (range, 250-300 g) were used as donors and recipients. In this study, we use a common technique for heterotopic cervical heart transplantation in rats, which shortens operation time, leading to a high average survival rate of receptor. Hearts from Wistar rats (n=20) were heterotopically transplanted into Lewis rats (n=20). Additional syngeneic hearts were transplanted from Lewis to Lewis (n=40). All animals were operated under anesthesia with chloral hydrate. After surgery, animals were maintained under the same comfortable conditions and fed the same food and water without any antibiotic. Grafts were harvested respectively at postoperative days (POD) 1, 3, 5, and 7. Technically successful transplantation is defined as the forceful beat of the transplant heart. The cardiac grafts rejection was considered complete by cessation of impulse. All surgical interventions and postoperative animal care were performed in accordance with the National Institutes of Health Guide-lines for the Care and Use of Laboratory Animals (National Research Council, 1996, USA) and were approved by the Chinese National Committee to the Use of Experimental Animals for Medical Purposes, Jiangsu Branch. All procedures were performed on animals in an unconscious state. All efforts were made to minimize the number of animals used and their suffering.
Western blot analysis
Western blot was prepared from allogeneic and syngeneic cardiac tissue at each time point after operation (n=5 for each time point). To obtain samples for western blot, the cardiac tissue were excised and snap frozen at –80 °C until use. Total protein was isolated from approximately 0.1 g of cardiac tissue at indicated time points. To prepare lysates, frozen heart samples were minced with eye scissors in ice. Total heart tissue protein were then homogenized in lysis buffer containing 1% NP-40, pH 7.5, 5 mmol/L EDTA, 50 mmol/L Tris, 1% SDS, 1% Triton X-100, 1% sodium deoxycholate, 10 mg/mL aprotinin, 1 mmol/L PMSF, and 1 mg/mL leupeptin, then micro centrifuge at 10,000 rpm and 4 °C for 20 min to collect the supernatant. After protein concentrations were determined with a Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA), the resulting supernatant was subjected to with SDS-polyacrylamide gel electrophoresis (PAGE). Proteins were transferred to polyvinylidine diflouride filter (PVDF) membranes (Millipore) by a transfer apparatus at 350 mA for 2.5 h. The membranes were blocked with 5% nonfat milk in Tris-buffered saline with Tween (TBST) at room temperature (RT) for 2 h. The filters were immediately rinsed three times in TBST and then incubated overnight with primary antibodies at 4 °C. Finally, the horseradish peroxidase-conjugated secondary antibody was added to filters for an additional 2 h, and the proteins were examined with an enhanced chemiluminescence detection system (ECL, Pierce Company, USA).
Immunohistochemistry
The syngeneic and allogeneic rats were deeply anesthetized and perfused through the AA with 500 mL of 0.9% saline, followed by 4% paraformaldehyde at different survival time points (n=5 for each time point). After the perfusion, the hearts of the allogeneic and syngeneic rats were removed and post-fixed in 4% paraformaldehyde for 4 h. The hearts were then subsequently steeped in 20% sucrose for three days and followed by 30% sucrose for another 3 days. Next, the hearts were embedded in OCT (opti-mum cutting temperature compound) compound for cryosectioning. The tissue was cut into 7-μm-thick sections and stored in a freezer at –20 °C. The sections were removed from the icebox, kept in an oven at 37 °C for 2 h, and washed three in 0.01 M PBS for 5 min. All of the sections were blocked with 10% goat serum with 1% (w/v) bovine serum albumin (BSA) and 0.3% Triton X-100 at RT for 2 h and was incubated overnight at 4 °C with anti-VRK1 antibody (anti-mouse, 1:50; Abgent), then by incubation in a biotinylated secondary antibody (Vector Laboratories, Burlin-game, CA, USA). Immunostaining was visualized with DAB (Diaminobenzidine, Vector Laboratories). Images were collected under a light microscope (40×) using a DFC 300 FX camera (Leica, DM 5000B; Leica CTR 5000; Germany). Same exposure time and light intensity were applied to all photographs.
Double immunofluorescent staining
The sections were removed from the icebox, kept in an oven at 37 °C for 2 h, and washed three in 0.01 M PBS (phosphate buffer solution) for 5 min. To avoid unspecific staining, the sections were blocked with 10% goat serum [3% (w/v) BSA and 0.05% Tween 20 and 0.1% Triton X-100] at RT for 2 h in order to avoid unspecific staining. Then the slides were incubated with polyclonal antibody specific for VRK1 (Santa Cruz, 1:50) and different markers as follows: active caspase-3 (Santa Cruz, 1:100), α-actinin (a marker of heart, Sigma, 1:100), VCAM-1 (a maker of rejection), CD4 (a maker of T cell) overnight at 4 °C. After was washing in PBS for three times, each time 15 min, a mixture of 4’,6-diamidino-2-phenylindole (DAPI) and Fluorescein isothiocyanate- (FITC-) and Cy3-conjugated secondary antibodies were added in a dark room and incubated for 2 h at 4 °C. Images were collected by Leica fluorescence microscope (Germany). Same exposure time and light intensity were applied to all photographs.
Statistical analysis
All of the data were analyzed with the Stata 7.0 statistical software. All values were expressed as the mean ± SEM. The significance of differences between the experimental groups was determined by a one-way analysis of variance (ANOVA) followed by the Tukey’s post hoc multiple comparison tests. P value of less than 0.05 was considered to be statistically significant. Each experiment consisted of at least five replicates per condition.
Results
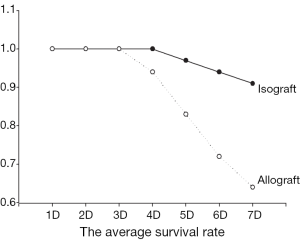
The average survival rate
The forceful beat of the transplant heart after 72 h was defined as technically successful transplantation. The average survival rate of heterotopic syngeneic heart transplantation is about 92.5%, and 88.5% in allograft (Figure 1), which showed no significant difference in syngeneic and allogeneic rats (92.5% vs. 88.5%, P>0.05).
The expression of VRK1 in allograft and isograft
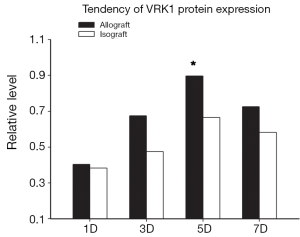
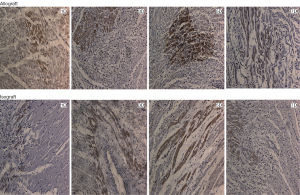
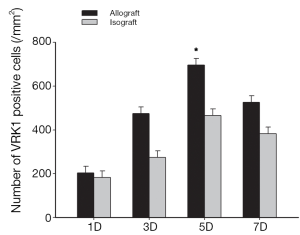
To examine the expression pattern of VRK1, western blot analysis and immunohistochemistry experiments were performed on the allograft and isograft at various time points. The VRK1 expression level was low in syngeneic cardiac tissue, but in allografts high expressions of VRK1 is increased observably after operation (Figure 2). Heart tissues expression of VRK1 both in allograft and isograft were increased quite markedly after POD5 (P<0.05) (Figure 3). In addition, the immunohistochemistry experiments also demonstrated that VRK1 staining was extensively expressed in the cardiomyocytes after heart transplantation (Figure 4). Compared with the syngeneic cardiac graft, the stainings of VRK1-positive cells were increased observably after operation in allografts (Figure 5). These data indicated that VRK1 protein level had a temporary change after heart transplantation.

The co-localization of VRK1 and α-actinin
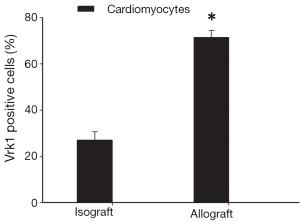
To further confirm the cell types expressing VRK1 in grafted cardiac tissues, double labeling immunofluorescent staining was performed. In the isograft tissues, the expression of VRK1 was low in cytoplasm of cardiomyocytes at POD5 (Figure 6). However, in the allograft tissue enhanced VRK1 immunocompetence was found in cytoplasm around the cardiomyocytes at POD5 (Figure 6). VRK1 expression was significantly increased in cardiomyocytes (P<0.05) at POD5 compared with the isograft group. To identify the proportion of myocardium-positive cells expressing VRK1, a minimum of 200 phenotype-specific marker positive cells were counted between the isograft and allograft groups (Figure 7).
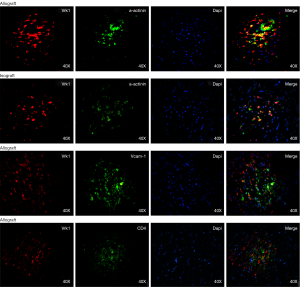
The relationship of VRK1 and inflammatory cells
Besides, we examined the relationship between VRK1 with inflammatory cells, aiming to exclude whether the immune response is responsible for VRK1. Double labeling immunofluorescent staining was performed with the following cell-specific markers: VCAM-1, CD4 (Figure 6). In the allograft tissues, the co-localization of VRK1 with CD4 was not observed at POD 5. The result of co-localization between VRK1 and VCAM-1 could not be observed (Figure 6). The results indicate that VRK1 don’t involving in immune rejection induced inflammatory cells infiltration after heart transplantation.
The co-localization of VRK1 and active-caspace-3
To understand whether VRK1 regulates the apoptosis of cardiomyocytes after allograft in murine, we examined the relationship between VRK1 and active caspase-3 after heart transplantation. Western blot analysis showed that the constitutive active caspase-3 level was low at POD1 in allograft, but increased at POD3 and reached a peak at POD5 (P<0.05) (Figure 8). The double labeling immunofluorescent staining was performed with the following cell-specific markers: VRK1, DAPI, active caspase-3 to assess the preliminary correlations between VRK1 and active caspase-3 in the allograft at POD5. Markedly, the co-localization of the two molecules increased more significantly in allograft (Figure 9).

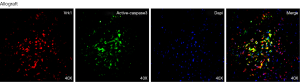
Discussion
Since the first abdominal heterotopic heart transplantation in the mouse was built in 1973 by Corry et al. (10), then Matsuura and colleagues published the cervical heterotopic heart transplantation in the mouse in 1991 (11) and many modified techniques for heterotopic cervical heart transplantation in rats (12-15). Heart transplantation remains the chief method for patients with incurable cardiac diseases (16). As we all known, after heterotopic heart transplantation it exist many pathologic changes: polymorphonuclear leukocyte (PMN)-mediated tissue damage, macrophage infiltrates, cardiac hypertrophy and so on (17,18). In addition, increasing evidence has intensively demonstrating the existence of apoptosis in cardiac tissue samples (19,20). Many researches have showed that cardiomyocytes apoptosis is popular in allograft (21,22), however, there is not a satisfactory method to ameliorate apoptosis.
In the human kinome, the VRK gene family is deemed a novel branch from the lineage which led to the group of CK. The human VRK proteins were originally identified by their homology with the B1R kinase of vaccinia virus, an early protein required for viral DNA synthesis and replication (23). VRK1 in proliferating tissues is highly expressed, such as tumor cell lines, and during the hematopoietic proliferative phase in murine embryo midgestation (23).
Susana and Pedro conclude that VRK1 is an up stream regulator of p53 that be longs to a new signaling pathway (24). Regulation of p53 levels plays a major role in determining the fate of a cell and its susceptibility to tumor development (7,25). These evidences showed that VRK1 can regulate cell proliferation in many tumors and resist cell apoptosis. However, the expression and significance of VRK1 after rat heart transplantation in domestic and international was not reported.
Therefore, whether VRK1 could regulate the cardiac cells from apoptosis in grafted heart remains an unknown question. According to the western blot, the expression of VRK1 is obviously expressed in allogeneic transplanted heart (Figures 2,3). But in isogeneic transplanted heart, the expression of VRK1 protein level was low (Figures 2,3). To determine the cellular localization of VRK1 in isogeneic and allogenic transplanted heart, we performed immunohistochemistry experiments with anti-VRK12 mouse monoclonal antibody. In the isogeneic transplanted heart, positive VRK1 staining was relatively low, however, in allogeneic transplanted heart the stainings of VRK1-positive cells were increased around the cardiomyocytes, especially at POD5 (Figures 4,5). These data indicated that VRK1 protein level had a temporary change after heart transplantation both in allogeneic and isogeneic transplanted heart.
In order to research the significance of VRK1 in cardiomyocytes after heart transplantation, we examined the relationship of VRK1 and inflammatory cells. In the allograft tissues, the co-localization of VRK1 with CD4 was not observed at POD5. The result of co-localization between VRK1 and VCAM-1 could not be observed (Figure 6). The results indicate that VRK1 don’t involving in immune rejection induced inflammatory cells infiltration after heart transplantation. To understand whether VRK1 regulates the apoptosis of cardiomyocytes after allograft in murine, we examined the relationship between VRK1 and active caspase-3 after heart transplantation. The result showed that active caspase-3 has a similar expression pattern with VRK1 (Figures 8,9). We propose VRK1 may regulate the cardiomyocytes apoptosis after heterotopic heart transplantation in murine.
Taken all, we showed that VRK1 expression in cardiomyocytes at allograft was higher than isograft cardiac tissue. VRK1 may serve as a novel therapeutic target for protecting ardiomyocytes after heart transplantation in human.
Acknowledgements
This work was supported by the National Natural Scientific Foundation of China Grant (No.31300902), the “Top Six Types of Talents” Financial Assistance of Jiangsu Province Grant (WS-059) and the Science Foundation of Nantong City Grant (K2010055).
Disclosure: The authors declare no conflict of interest.
References
- Burch M, Aurora P. Current status of paediatric heart, lung, and heart-lung transplantation. Arch Dis Child 2004;89:386-9. [PubMed]
- Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2006;113:e85-151. [PubMed]
- Taylor DO, Edwards LB, Mohacsi PJ, et al. The registry of the International Society for Heart and Lung Transplantation: twentieth official adult heart transplant report--2003. J Heart Lung Transplant 2003;22:616-24. [PubMed]
- John R, Chen JM, Weinberg A, et al. Long-term survival after cardiac retransplantation: a twenty-year single-center experience. J Thorac Cardiovasc Surg 1999;117:543-55. [PubMed]
- Shi J, Qian S, Meng X, et al. Reliability of intramyocardial electrogram for the noninvasive diagnosis of acute allograft rejection after heart transplantation in rats. J Thorac Dis 2014;6:126-33. [PubMed]
- Klerkx EP, Lazo PA, Askjaer P. Emerging biological functions of the vaccinia-related kinase (VRK) family. Histol Histopathol 2009;24:749-59. [PubMed]
- Vega FM, Sevilla A, Lazo PA. p53 Stabilization and accumulation induced by human vaccinia-related kinase 1. Mol Cell Biol. 2004;24:10366-80. [PubMed]
- Sevilla A, Santos CR, Barcia R, et al. c-Jun phosphorylation by the human vaccinia-related kinase 1 (VRK1) and its cooperation with the N-terminal kinase of c-Jun (JNK). Oncogene 2004;23:8950-8. [PubMed]
- Nezu J, Oku A, Jones MH, et al. Identification of two novel human putative serine/threonine kinases, VRK1 and VRK2, with structural similarity to vaccinia virus B1R kinase. Genomics 1997;45:327-31. [PubMed]
- Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation 1973;16:343-50. [PubMed]
- Matsuura A, Abe T, Yasuura K. Simplified mouse cervical heart transplantation using a cuff technique. Transplantation 1991;51:896-8. [PubMed]
- Vespignani R, Ruzza A, Czer LS, et al. High definition optical system for microsurgical heterotopic heart transplantation in rats. Transplant Proc 2012;44:1404-6. [PubMed]
- Huang X, Chen D, Chen L. A modified model of cervical heterotopic cardiac transplantation for chronic rejection research. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2008;22:1508-10. [PubMed]
- Yoshida Y, Hatayama N, Seki K. Study on the preservation with CO (PCO = 200-2,000 hPa), resuscitation, and heterotopic transplantation of an isolated rat heart. Cell Transplant 2009;18:535-40. [PubMed]
- Ma Y, Wang G. Comparison of 2 heterotopic heart transplant techniques in rats: cervical and abdominal heart. Exp Clin Transplant 2011;9:128-33. [PubMed]
- Wu W, Qiu Q, Wang H, et al. Nrf2 is crucial to graft survival in a rodent model of heart transplantation. Oxid Med Cell Longev 2013;2013:919313.
- El-Sawy T, Miura M, Fairchild R. Early T cell response to allografts occurring prior to alloantigen priming up-regulates innate-mediated inflammation and graft necrosis. Am J Pathol 2004;165:147-57. [PubMed]
- Qian Z, Wasowska BA, Behrens E, et al. C6 produced by macrophages contributes to cardiac allograft rejection. Am J Pathol 1999;155:1293-302. [PubMed]
- Shaw SM, Critchley WR, Puchalka CM, et al. Brain natriuretic peptide induces CD8+ T cell death via a caspase 3 associated pathway--implications following heart transplantation. Transpl Immunol 2012;26:119-22. [PubMed]
- Sánchez-Lázaro IJ, Almenar-Bonet L, Romero-Pelechano A, et al. Serum markers of apoptosis in the early period of heart transplantation. Biomarkers 2012;17:254-60. [PubMed]
- Cristóbal C, Segovia J, Alonso-Pulpón LA, et al. Apoptosis and acute cellular rejection in human heart transplants. Rev Esp Cardiol 2010;63:1061-9. [PubMed]
- Du JF, Shen XF, Ji XQ, et al. Apoptosis of peripheral T cells in rodent cardiac allograft recipients induced by donor-specific transfusion with impaired inducible costimulator/B7 homologous protein allorecognition. Transplant Proc 2013;45:564-8. [PubMed]
- Sevilla A, Santos CR, Vega FM, et al. Human vaccinia-related kinase 1 (VRK1) activates the ATF2 transcriptional activity by novel phosphorylation on Thr-73 and Ser-62 and cooperates with JNK. J Biol Chem 2004;279:27458-65. [PubMed]
- Lopez-Borges S, Lazo PA. The human vaccinia-related kinase 1 (VRK1) phosphorylates threonine-18 within the mdm-2 binding site of the p53 tumour suppressor protein. Oncogene 2000;19:3656-64. [PubMed]
- Valbuena A, Vega FM, Blanco S, et al. p53 downregulates its activating vaccinia-related kinase 1, forming a new autoregulatory loop. Mol Cell Biol 2006;26:4782-93. [PubMed]


