Sodium tanshinone IIA silate as an add-on therapy in patients with unstable angina pectoris
Introduction
Unstable angina pectoris (UAP) as a subclass of acute coronary syndrome (ACS), is one of important causes of death in cardiovascular diseases (1-3). A common occurrence in ACS patients may be the cardiac instability and rupture of atherosclerotic plaques (4,5). Several studies have demonstrated the involvement of inflammatory response in these events and the subsequent thrombus formation (6-9).
Sodium tanshinone IIA silate (STS), a tanshinone IIA (Tan IIA) derivative, is an active, lipid-soluble component isolated from Salvia miltiorrhiza (10) which has been shown to improve blood circulation, dilate coronary arteries, reduce heart rate, increase myocardial contractility, and relieve hypoxia-induced myocardial disorders (11,12). STS exhibits a range of favorable cardiovascular actions like anticoagulation, tissue repair, and blood lipid-lowering (13,14). In addition, studies also suggested the role of STS in modulating inflammatory response associated with the process of myocardial infarction (MI), endothelium injury, atherosclerosis, and cardiovascular hypertrophy (2-5,15). However, so far the data remain limited regarding the profiles of inflammatory factors in ACS patients receiving STS. We hypothesized that STS could relieve inflammation response and thus improve the clinical efficacy in patients with UAP.
This study was conducted to investigate whether STS as an add-on treatment to conventional treatment may provide additional benefits for UAP patients and is associated with changes in profiles of serum inflammatory factors, such as monocyte chemotactic protein 1 (MCP-1), tumor necrosis factor alpha (TNF-α), peroxisome proliferator-activated receptor (PPAR-γ), high-sensitivity C-reactive protein (hs-CRP), thereby improving the clinical curative effect in this subset of patients.
Patients and methods
Study population
Between August 2012 and April 2014, a cohort of consecutive patients with UAP hospitalized at Department of Cardiology, The Second Affiliated Hospital of Nanjing Medical University, were included in this study. The diagnosis of UAP was based on conventional coronary angiography or computed tomographic angiography, following the diagnostic criteria jointly established by American Heart Association/American College of Cardiology (ACCF/AHA) (16). Exclusion criteria were as follows: (I) a history of cerebral hemorrhage or acute cerebral infarction in the previous 3 months; (II) a history of surgery and trauma in the previous 6 months; (III) severe left ventricular dysfunction (EF <30%); (IV) severe hepatic and renal dysfunctions; (V) deep vein thrombosis of the lower extremity or pulmonary embolism; and (VI) signs of acute or chronic infections. The patients were randomized into the treatment group and the control group based on a computer-generated program. Baseline characteristics of these patients, including blood pressure, blood glucose, serum lipids, smoking history, co-morbidities, and other general demographics were recorded. This study was approved by the Ethics Committee of The Second Affiliated Hospital of Nanjing Medical University. All patients who participated in the study gave informed consent.
Treatments
After admission, all patients were given conventional treatment, which included oxygen inhalation, bed rest, ECG monitoring and oral aspirin or clopidogrel, as well as administration of angiotensin converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB), lipid-lowering agents, β-blockers, and calcium ion antagonists, according to patients’ conditions. The treatment group was given additional intravenous STS (0.06 mg in 250 mL normal saline, once daily). The duration of treatment was 2 weeks. Throughout the study, all patients were allowed to take oral nitroglycerin for relieving agina attacks as needed. Blood and urine samples were analyzed, and liver and kidney function, electrocardiogram (ECG), dynamic ECG, fasting blood glucose, blood lipids were measured at baseline and at the end of the 2-week treatment. The clinical events (such as ST segment changes), the frequency and duration of angina pectoris attacks, and the dosage of nitroglycerin were also recorded. Adverse reactions related to use of STS were observed and noted, if any.
Measurements of blood sample
Fasting blood samples (3 mL) were collected in anticoagulant tubes from both groups of patients at baseline and after 2 weeks of treatments. The samples were centrifuged at 3,000 r/min for 15 min, allowed to sit for 3 h, and then the serum was isolated and stored at –70 °C. Serum levels of MCP-1, TNF-α, and PPAR-γ were measured by enzyme-linked immunosorbent (ELISA), according to the manufacturer’s instructions (R&D Systems, Minneapolis, USA). Serum hs-CRP was detected by using the immune turbidity method (Orion Diagnostica, Uusimaa, Finland).
Evaluation of therapeutic efficacy
At the end of 2-week treatment, we evaluated the therapeutic efficacy in all patients, which was categorized as: (I) significantly effective—no angina attacks or up to 80% reduction in the duration of angina attacks; ST segment returned to normal or nearly normal; >80% reduction in the total time of myocardial ischemia as indicated by dynamic ECG; (II) effective—50% to 80% reduction in the frequency or duration of angina attacks; ST depression reduced by more than 0.1 mV; 50% to 80% reduction in the total time of myocardial ischemia; and (III) ineffective—less than 50% reduction in the frequency or duration of angina attack; ST depression did not obviously improved; <50% reduction in total time of myocardial ischemia on ECG.
Statistical analysis
Statistical analyses were performed by using the Statistical Package for the Social Sciences Ver. 16.0 for Windows (SPSS, Chicago, IL, USA). Numerical values were expressed as means ± standard deviation. Between-group comparison was performed by ANOVA or Chi-square tests where applicable. P<0.05 was considered statistically significant.
Results
Demographics and clinical characteristics of the patients
A total of 80 UAP patients who fulfilled the inclusion criteria and passed the exclusion criteria were included in this study, comprising 22 males and 18 females in the treatment group (n=40; aged, 39 to 74 years old; mean, 63.5±12.3 years old) versus 25 males and 15 females in the control group (n=40; aged, 41 to 75 years old; mean, 66.3±9.2 years old). The clinical characteristics of the patients were shown in Table 1. There were no significant differences in age, sex, blood pressure, fasting blood glucose, smoking history, high-risk factors and use of conventional medications between the two groups (P>0.05).
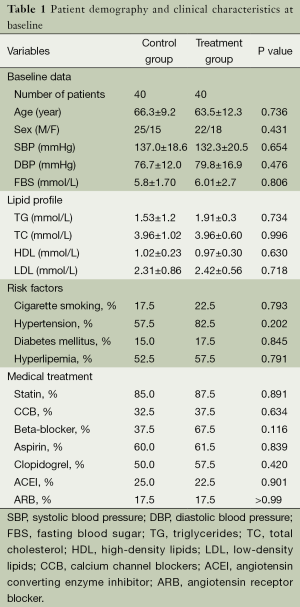
Full table
Treatment outcomes
Evaluation of the therapeutic efficacy identified 20 cases with significant effective response and 19 with effective response contributing to an overall effectiveness rate of 97.5% in the treatment group, compared with 12 cases with significant effective response and 20 with effective response contributing to an overall effectiveness rate of 80.0% in the control group (P<0.05). The nitroglycerin dosages after the 2-week treatment in both groups were significantly reduced from baseline. Noticeably, the reduction in nitroglycerin dosage was greater in the treatment group (P<0.01; Table 2). Moreover, there were no adverse events related to use of STS in the treatment group.
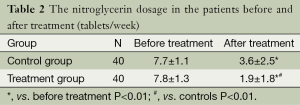
Full table
Changes in serum inflammatory cytokines
At the end of 2-week study, the serum levels of MCP-1, TNF-α, and hs-CRP in all subjects were significantly reduced from baseline (P<0.01), and the reduction in all these inflammatory factors was more evident in the treatment group than in the control group (P<0.05). In contrast, the serum level of PPAR-γ was elevated in both groups after treatment compared with baseline (P<0.01). The increase in PPAR-γ seemed slightly greater in patients who received additional STS than the controls, though, the difference did not reach a level of significance (P=0.231) (Table 3).
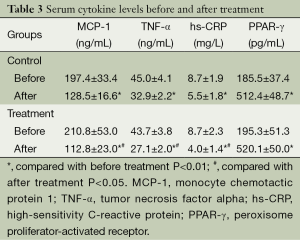
Full table
Discussion
Over the past decade, UAP has become an important subtype of ACS contributing to high morbidity and mortality, and therefore attracting more research attention. A growing body of evidence suggests that UAP is associated with local and systemic activation of the immune system (17,18). Studies show that UAP is a complicated pathological progression involving the interaction of many factors. Plasma levels of several inflammation markers have been found to be associated with future cardiovascular risk in patients with UAP. Recently, multi-target and multi-link agents derived from herbal Chinese medicine are widely discussed as to their significant role in the systematic intervention of UAP. Hence, it would be a topic of research interest whether STS with favorable inflammation modulating actions used as additional treatment could provide clinical benefits and thus improve the therapeutic efficacy in patients with UAP.
In this study, 80 patients with UAP were recruited to investigate the efficacy of STS on several inflammatory cytokines in order to provide evidence for its clinical use in this disease. Our results showed that STS, in combination with conventional treatment, may reduce the serum levels of MCP-1, TNF-α and hs-CRP, and elevate the level of PPAR-γ in patients with UAP.
Previous research has found that STS may exhibit myocardial protection against ischemia/reperfusion by inhibiting TNF-α through a positive feedback of the NF-κB/TNF-α pathways, suggesting an anti-inflammatory activity of STS (19,20). Furthermore, STS has also been shown to exert some anti-inflammatory effects by inhibiting inducible NOS (iNOS) gene expression and NO production, as well as inhibiting inflammatory cytokine (IL-1β, IL-6, and TNF-α) expression (21). These findings might be responsible for the mechanisms underlying the anti-inflammatory activity of STS. We also found that the levels of several serum biomarkers, including TNF-α, MCP-1 and hs-CRP, were decreased to a greater magnitude in the STS group as compared to controls (P<0.05). PPAR-γ is a type II nuclear receptor that helps decrease the inflammatory cytokine response in many cardiovascular cells (22-25). Elevation of serum PPAR-γ is therefore inhibitory against TNF-α, MCP-1 and hs-CRP, and may help further relieve of systemic inflammation. Our findings suggested that there was also a slightly greater increase in the serum PPAR-γ levels among patients given additional STS, although the difference did not reach level of significance, possibly owing to insufficient sample size. Further studies with inclusion of large patient series are warranted to validate our results.
Our results also showed that STS combined with conventional medications can improve the symptoms of UAP and lead to less use of nitroglycerin compared with the conventional treatment alone. The difference may result from the anti-inflammatory effects of STS. In addition, the add-on STS therapy was tolerable and safe in all patients given the treatment.
Several limitations in this study should be acknowledged. Firstly, this was a single-center study. Robust evidence with the benefits of STS in UAP patients would require on-going collaboration between multiple institutions in the future. Secondly, STS was used as an add-on therapy rather than a monotherapy in this study. The unique efficacy of STS could not be addressed with our observation, and would warrant subsequent research. Thirdly, the sample size in this study may not be fully adequate to shed light on the clinical benefits of STS.
Nevertheless, the present study may be an early attempt to explore the therapeutic value of STS as a derivative of herbal Chinese medicine drug in clinical practices. Although future validation needed, our results showed that STS in combination with conventional treatment may be associated with better outcomes in patients with UAP.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kubková L, Spinar J, Pávková Goldbergová M, et al. Inflammatory response and C -reactive protein value in patient with acute coronary syndrome. Vnitr Lek 2013;59:981-8. [PubMed]
- Napoleão P, Santos MC, Selas M, et al. Variations in inflammatory markers in acute myocardial infarction: a longitudinal study. Rev Port Cardiol 2007;26:1357-63. [PubMed]
- Garg A, Bansal A, Bhuyan S, et al. Paraplegia during coronary artery bypass graft surgery caused by bilateral anterior cerebral artery territory infarction. Ann Transl Med 2014;2:49. [PubMed]
- Pomianowski P, Elefteriades JA. The genetics and genomics of thoracic aortic disease. Ann Cardiothorac Surg 2013;2:271-9. [PubMed]
- Cerrato E, D'Ascenzo F, Biondi-Zoccai G, et al. Acute coronary syndrome in HIV patients: from pathophysiology to clinical practice. Cardiovasc Diagn Ther 2012;2:50-5. [PubMed]
- Searle J, Danne O, Müller C, et al. Biomarkers in acute coronary syndrome and percutaneous coronary intervention. Minerva Cardioangiol 2011;59:203-23. [PubMed]
- Yang GJ. Antiplatelet therapy in patients with hypertension. J Transl Intern Med 2013;1:28-31.
- Shang Q, Xu H, Huang L. Tanshinone IIA: A Promising Natural Cardioprotective Agent. Evid Based Complement Alternat Med 2012;2012:716459.
- Wang J, Lu W, Wang W, et al. Promising therapeutic effects of sodium tanshinone IIA sulfonate towards pulmonary arterial hypertension in patients. J Thorac Dis 2013;5:169-72. [PubMed]
- Raja SG. Sodium tanshinone IIA sulfonate for pulmonary arterial hypertension: emerging therapeutic option. J Thorac Dis 2013;5:114-5. [PubMed]
- He WF, Lu Z, Zhang QB. Research progress on Tanshinone in pharmacological mechanism of cardiovascular protection. China Medical Herald 2013;10:34-8.
- Cheng F, Yang LF. Clinical Observation on Sulfotanshinone Sodium Injection treating angina pectoris. Northern Pharmacology 2013;10:56-7.
- Jin UH, Suh SJ, Chang HW, et al. Tanshinone IIA from Salvia miltiorrhiza BUNGE inhibits human aortic smooth muscle cell migration and MMP-9 activity through AKT signaling pathway. J Cell Biochem 2008;104:15-26. [PubMed]
- Fang ZY, Lin R, Yuan BX, et al. Tanshinone IIA downregulates the CD40 expression and decreases MMP-2 activity on atherosclerosis induced by high fatty diet in rabbit. J Ethnopharmacol 2008;115:217-22. [PubMed]
- Fang ZY, Lin R, Yuan BX, et al. Tanshinone IIA inhibits atherosclerotic plaque formation by down-regulating MMP-2 and MMP-9 expression in rabbits fed a high-fat diet. Life Sci 2007;81:1339-45. [PubMed]
- Ren ZH, Tong YH, Xu W, et al. Tanshinone II A attenuates inflammatory responses of rats with myocardial infarction by reducing MCP-1 expression. Phytomedicine 2010;17:212-8. [PubMed]
- Feng Y, Zhang JC, Xi RX. Clinical significance of inflammation factors in acute coronary syndrome from pathogenic toxin. Chin J Integr Med 2009;15:307-12. [PubMed]
- Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2012;60:645-81. [PubMed]
- Lu Y, Li L, Yan H, et al. Endothelial microparticles exert differential effects on functions of Th1 in patients with acute coronary syndrome. Int J Cardiol 2013;168:5396-404. [PubMed]
- Gökçe M, Erdöl C, Orem C, et al. Inflammation and immune system response against unstable angina and its relationship with coronary angiographic findings. Jpn Heart J 2002;43:593-605. [PubMed]
- Honoré PM, Spapen H. Embracing western and Chinese research and clinical knowledge: We take up the translational gauntlet! J Transl Intern Med 2013;1:1-2.
- Wu WY, Wang WY, Ma YL, et al. Sodium tanshinone IIA silate inhibits oxygen-glucose deprivation/recovery-induced cardiomyocyte apoptosis via suppression of the NF-κB/TNF-α pathway. Br J Pharmacol 2013;169:1058-71. [PubMed]
- Ripley DP, Motwani M, Plein S, et al. Established and emerging cardiovascular magnetic resonance techniques for the assessment of stable coronary heart disease and acute coronary syndromes. Quant Imaging Med Surg 2014;4:330-44. [PubMed]
- Fan GW, Gao XM, Wang H, et al. The anti-inflammatory activities of Tanshinone IIA, an active component of TCM, are mediated by estrogen receptor activation and inhibition of iNOS. J Steroid Biochem Mol Biol 2009;113:275-80. [PubMed]
- Ding Y, Yang KD, Yang Q. The role of PPARδ signaling in the cardiovascular system. Prog Mol Biol Transl Sci 2014;121:451-73. [PubMed]


