Video-assisted thoracic surgery resection of rib osteophytes
Introduction
Bone osteophytes mostly occur in the spine and weight-bearing joints (1,2). Bone osteophytes are a result of degeneration due to biomechanical changes associated with aging, trauma and accumulation of external strain (3-5). Related conditions will occur when bone spurs compress the surrounding tissue and cause injury in motion and at rest, leading to a series of pathological changes. The patient reported in this study had multiple spurs and was also present with spontaneous hemothorax. His spurs were also close to the junction of cartilage and ribs.
Clinical data
The 19-year-old boy was admitted due to “left chest pain for two days”. The patient had left chest pain with no obvious causes two days ago, which was associated with paroxysmal irritation, cough, and a small amount of white sputum. There was chest tightness and shortness of breath after exertion, which could be relieved by rest. He received examination in another hospital. The chest X-ray suggested “left pleural effusion”, so left pleural puncture was done, draining out bloody pleural effusion of about 150 mL. Later, the patient’s symptoms improved slightly. He was admitted to our hospital on February 7, 2013 for further diagnosis and treatment. He had undergone surgery in 2007 due to “lower limb osteochondroma”. There was no history of trauma. Admission examination: weak breath sounds in the left lung, with dullness upon percussion. Blood tests: WBC, 8.87×109/L; erythrocytes, 3.69×1012/L; neutrophils, 7.01×109/L; lymphocytes, 1.25×109/L; platelets, 175×109/L; hemoglobin, 76 g/L; hematocrit, 30.5%. Chest X-ray and CT scans both showed left pleural effusion and lung tissue compression. After admission, the patient’s hemoglobin decreased progressively, and he underwent thoracoscopic hemothorax removal, lung laceration repair, and spur resection.
Preoperative assessment
Left pleural puncture was done under ultrasound positioning to drain about 780 mL of non-coagulating blood. A repeat ultrasonic examination suggested that there was still a visible liquid dark area in the anterior part of the left costophrenic angle, in which insufficiently inflated lung tissue could be seen. Blood tests: 76 g/L, hematocrit: 30.5%, hemoglobin decreased significantly, suggesting that there were still effusion and blood clots in the left chest cavity. Without surgical treatment, hemorrhagic shock and parcel hemothorax or even empyema could be the result. Since the patient was young with favorable cardiac and pulmonary function as assessed before surgery, and there was no abnormality found in the intrathoracic angiography, he could undergo double-lumen endotracheal intubation and surgery. Because there was no definite diagnosis before surgery, the source of bleeding remained unknown and the surgery was exploratory. To minimize surgical injury, we used thoracoscopic exploration, and the appropriate surgical treatment would be decided on based on the result.
Anesthesia and patient position
The patient was put in a lateral position with arms extended to 90 degrees, and elbows bent at 90 degrees. To protect the intercostal neurovascular bundle, the operating table was folded to maximize the intercostal space.
General anesthesia was performed through intubation using the double lumen endotracheal tube, making separate ventilation possible for both lungs. This allowed deflation of the lung on the one side while maintaining contralateral lung ventilation. During the surgery, the intercostal nerve was frozen rather than using epidural analgesia to avoid hypotension. Meanwhile, blood transfusion was applied to maintain a stable blood pressure.
Procedure
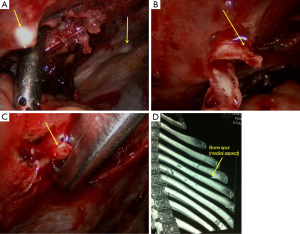
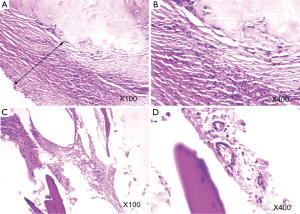
A 1.2-cm incision was made in the 7th intercostal space at the left axillary midline to insert the trocar and thoracoscope. A 1.2-cm working port was made each in the 3th intercostal space at the left anterior axillary line and 6th intercostal space at the left posterior axillary line. Exploration showed about 700 mL of blood in the chest cavity, adhesion of the left upper lingular segment to the anterior chest wall, and a large amount of blood clots in the chest. After separation of the adhesion, removal of blood and flushing of the chest, a second exploration revealed multiple beaded bony protrusions of varying sizes along the junction between the 2nd, 4th, 6th and 7th ribs and the cartilage on the left side. Hyperplasia of the covering pleural tissue was noticed. The junction between the 4th rib and the cartilage was in a tapered spur shape of about 1.0 cm long (Figure 1A), covered with full pleura. The end of the bone spur penetrated the left upper lingular segment, leaving an approximately 0.5 cm wound with active bleeding. The pleura covering the spurs were separated (Figure 1B). The hyperplastic spurs at the left 4th rib and 2nd rib were removed using a plate rongeur (Figure 1C). The blood was stopped and the chest rinsed. A closed chest dual drainage was placed, and the incision was sutured. The preoperative CT images were used for three-dimension rib reconstruction, in which the spurs were clearly revealed (Figure 1D). After surgery, the rib osteophytes were confirmed by pathology (Figure 2).
Postoperative management
After the surgery, the patient received routine chest X-ray and blood tests to ensure the amount of fluid was within 1.5 L. These measures can avoid tracheal and pulmonary inhalation of foreign bodies and pulmonary edema after lung resection. Painkillers and antibiotics were administered according to the guidelines. The chest drainage was removed three days later.
Comment
Since the late 1990s, the video-assisted thoracoscopic surgery (VATS) technique has been applied by domestic and foreign physicians in the treatment of lung, esophagus, mediastinum, progressive hemothorax, blood coagulation and other types of chest diseases (6-9). To minimize surgical injury, we used thoracoscopic surgery resection of rib osteophytes. The characteristics of this case included: sudden chest pain, pleural effusion, progressive hemoglobin decrease; cough without fever, pleural effusion, with pulmonary imaging that could not be simply explained by lung infections and pleurisy necessitating other diagnoses; and diagnostic thoracentesis conducive to diagnosis of hemothorax and differential diagnosis from pleural effusion. The following should be taken into account for the diagnosis of spontaneous hemothorax: whether there was tear of intrathoracic adhesions, congenital hemangioma in vascular malformation, tumors, parasites and systemic diseases. Rib construction helped reveal the presence of bone spurs. If the rib bone spurs were clearly diagnosed, caution should be made to clarify whether the adjacent organs were injured, such as the lungs, diaphragm, heart and blood vessels, leading to pneumothorax, hemothorax, cardiac tamponade and other symptoms. The case has spurs near the junction of cartilage and ribs. Whether this site is prone to the occurrence of this condition needs to be clarified with more cases. In particular, this patient had many forming osteophytes in such areas. Therefore, follow-up is needed to observe whether these develop into spurs that may cause injury.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Coumans JV, Neal JB, Grottkau BE, et al. Giant thoracic osteophyte: a distinct clinical entity. J Clin Neurosci 2014;21:1599-602. [PubMed]
- Podsiadlo P, Cicuttini FM, Wolski M, et al. Trabecular bone texture detected by plain radiography is associated with an increased risk of knee replacement in patients with osteoarthritis: a 6 year prospective follow up study. Osteoarthritis Cartilage 2014;22:71-5. [PubMed]
- Cağlar E, Sahin G, Ogur T, et al. Quantitative evaluation of hyaline articular cartilage T2 maps of knee and determine the relationship of cartilage T2 values with age, gender, articular changes. Eur Rev Med Pharmacol Sci 2014;18:3386-93. [PubMed]
- Aldrian S, Zak L, Wondrasch B, et al. Clinical and Radiological Long-term Outcomes After Matrix-Induced Autologous Chondrocyte Transplantation: A Prospective Follow-up at a Minimum of 10 Years. Am J Sports Med 2014;42:2680-8. [PubMed]
- Hada S, Kaneko H, Sadatsuki R, et al. The degeneration and destruction of femoral articular cartilage shows a greater degree of deterioration than that of the tibial and patellar articular cartilage in early stage knee osteoarthritis: a cross-sectional study. Osteoarthritis Cartilage 2014;22:1583-9. [PubMed]
- Walker WS, Carnochan FM, Mattar S. Video-assisted thoracoscopic pneumonectomy. Br J Surg 1994;81:81-2. [PubMed]
- Walker WS, Codispoti M, Soon SY, et al. Long-term outcomes following VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J Cardiothorac Surg 2003;23:397-402. [PubMed]
- Shigemura N, Akashi A, Funaki S, et al. Long-term outcomes after a variety of video-assisted thoracoscopic lobectomy approaches for clinical stage IA lung cancer: a multi-institutional study. J Thorac Cardiovasc Surg 2006;132:507-12. [PubMed]
- Wright GM. Video-assisted thoracoscopic pulmonary resections - The Melbourne experience. Ann Cardiothorac Surg 2012;1:11-5. [PubMed]


