Chronic effects of air pollution on respiratory health in Southern California children: findings from the Southern California Children’s Health Study
Introduction
Adverse health effects of exposures to regulated air pollutants have been widely studied (1-6). Other pollutants including volatile organic compounds and ultrafine particles may also have adverse health effects (7), but these pollutants have not been as extensively studied. There is growing evidence for adverse respiratory health effects from ambient air pollutants and near-source local air pollutants such as automobile tailpipe emissions, a major source of air pollution in Southern California and many regions in the world. Recent studies have shown that both ambient air pollutants and near source exposure to traffic-related pollutants are associated with increased incidence of asthma (8-11), lung function deficits (12-14), and airway inflammation (15,16). Traffic-related combustion tailpipe emissions contain high concentrations of reactive gases and high concentrations of ultrafine particles among other toxic compounds. It should be noted that levels of the most toxic of these combustion products are not regulated in the current criteria framework, although the regulated ambient air pollutant levels decreased over the last decades (17).
Because children are more sensitive to the effects of air pollution than adults, due to rapidly growing and developing lungs and immune systems, research about the long-term impact of air pollution on the growth of lung function and respiratory illnesses is important to guide air pollution regulation and early prevention of respiratory diseases in the future. The Children’s Health Study (CHS) is one of the largest and most comprehensive investigations of the long-term consequences of air pollution on the respiratory health of children. The CHS has also studied the effects of air pollution on genetic and epigenetic variations in genes in oxidative/nitrosative stress pathway, and how the genetic and epigenetic variations in this pathway influence respiratory health outcomes. Results from the CHS have shown that both ambient air pollution (8,14,16,18-22) and traffic-related pollution (3,9,13,15) have adverse health effects. Additionally, children’s vulnerability to air pollution may be increased by higher level of parental stress (23), inadequate antioxidant defenses including low levels of vitamins A and C (24), and variations in the expression or function of antioxidant and inflammatory genes, such as glutathione-S-transferases (GSTs) (25-27), arginases (ARG1 and ARG2) (28), and tumor necrosis factor-α (TNF-α) (29). In addition to the findings reviewed in 2003 (30), we will summarize more recent findings from the CHS to highlight the heavy burden to children’s respiratory health of current air pollution levels, even though these levels are often below national air quality standard.
The Children’s Health Study (CHS)
The CHS study design has been described in detail in previous publications (13,30-33). Briefly, more than 11,000 school children were selected from classrooms in 16 communities in multiple waves of subject recruitment starting in 1993 to maximize the differences in regional air pollution concentrations and mixtures (Table 1). Beginning from study entry and continuing until high school graduation, yearly questionnaires assessed the development of respiratory symptoms and current activity patterns. Lung function was measured annually through spirometry. School absences were actively ascertained to evaluate the effects of pollution on acute respiratory illnesses. Outdoor concentrations of ozone (O3), particulate matter (PM) of less than 2.5 µm and less than 10 µm aerodynamic diameter (PM2.5 and PM10, respectively), and nitrogen dioxide (NO2) were measured continuously at central monitoring stations within each community. Several metrics of traffic-related pollution have been used, including (I) proximity of the residence to the nearest freeway or roadway; (II) average number of vehicles traveling within 150 m of the residence each day; (III) model-based estimates of traffic-related air pollution at the residence or school derived from dispersion models (CALINE) (8,12,34) and land-use regression exposures models (13).
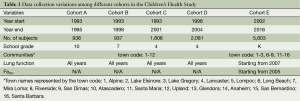
Full table
For the cohort of kindergarten and first grade student recruited in 2003 to study the relationship between air pollution and airway inflammation (Cohort E, Table 1), exhaled nitric oxide (FeNO) was collected using both an offline breath collection technique according to American Thoracic Society (ATS) guidelines [ATS 1999; ATS/European Respiratory Society (ATS/ERS) 2005] in the initial years of the study, and an online FeNO collection in subsequent study years (32).
Participants provided DNA beginning in 1998 using standard buccal cell collection procedures (35). Genomic DNA was isolated using a Puregene DNA isolation kit (Gentra Systems, Minneapolis, MN). Each genotype was validated by using PCR/restriction fragment length polymorphism methods (36). A nested case-control sample of 769 asthmatics and 1007 controls, who were either Hispanic white (n=817) or non-Hispanic white (n=959), were selected into CHS genome-wide association study. The genotyping was performed at the USC Epigenome Center using the Illumina HumanHap550, HunmanHap550-Duo or Human610-Quad BeadChip microarrays.
Main findings
Air pollution associations with asthma occurrence
Ambient air pollution has been associated with asthma prevalence and incidence in the CHS. In Cohort C,D, higher local NO2 concentrations were associated with higher asthma prevalence [odds ratio (OR), 1.83; 95% confidence interval (CI): 1.04-3.22; per interquartile range (IQR) =5.7 ppb NO2] after adjusting for sex, race, Hispanic ethnicity, cohort, and community (8) and higher risk of new-onset asthma [hazard ratio (HR), 1.29; 95% CI: 1.07-1.56; per IQR of 6.2 ppb NO2] after adjusting for Hispanic ethnicity, medical insurance, cohort, community, and relative humidity (9). In Cohort A-D, Regional O3 was associated with asthma incidence, but this association was modified by exercise (11). Specifically, the relative risk of asthma incidence associated with high regional O3 was three times higher among children playing three or more team sports, compared to children playing no sports after adjusting for ethnicity and community with baseline strata for age and sex (OR, 3.3; 95% CI: 1.9-5.8). A statistically significant positive association between number of team sports played and asthma incidence was observed only in communities with high O3 (means of O3 concentrations in high and low pollution communities =59.6 ppb and 40.0 ppb, respectively) (OR, 1.4; 95% CI: 1.0-2.1). However, in the subsequent Cohort E, regional NO2 and O3 did not appear to be associated with asthma incidence after additionally adjusting for non-freeway traffic-related pollution at home and school (HR, 1.37; 95% CI: 0.69-2.71; and HR, 1.01; 95% CI: 0.49-2.11, respectively) (10).
Across CHS cohorts, several metrics of traffic-related pollution have shown adverse effects on asthma prevalence and incidence, independent of regional ambient air pollution. In Cohort C and D, the risk of life-time asthma was 1.9-fold among children with closer residential distance to a freeway (below 25th percentile) compared to children with farther residential distance from a freeway (above 75th percentile) after adjusting for sex, race, Hispanic ethnicity, cohort, and community (OR, 1.89; 95% CI: 1.19-3.02) (8). An IQR [2.3 ppb nitrogen oxide (NOx)] increase of CALINE estimated freeway-related NOx was associated with more than 2-fold increased risk of lifetime asthma (OR, 2.22; 95% CI: 1.36-3.63) (8). In Cohort A-D, children with residences within 75 m of a major roadway had a 29% increased risk of lifetime asthma and a 50% increased risk of prevalent asthma after adjusting for age, sex, race community, and language of the questionnaire (English/Spanish) (3). In Cohort E, after adjustment for race/ethnicity and for baseline hazards strata of age at study entry and sex, and random effects of school and community, an IQR (8 ppb NOx) increase in CALINE estimated non-freeway traffic-related pollutions near home and school were both associated with a 1.5-fold increased risk of new-onset asthma, and these results were robust to adjustment for ambient NO2 (OR, 1.46; 95% CI: 1.16-1.84) for home; and OR, 1.45; 95% CI: 1.03-2.06 for school) (10). Recent results further suggested that the effect of traffic-related pollution on the risk of new-onset asthma can be modified by parental stress levels. After adjusting for race/ethnicity and community with baseline strata for age and sex, an IQR increase of non-freeway traffic-related pollution (21 ppb of NOx) was associated with a 1.5 times (HR, 1.51; 95% CI: 1.16-1.96) higher hazard of incident asthma for children with high parental stress versus a 1.1 times (HR, 1.05; 95% CI: 0.74-1.49) higher hazard of incident asthma for children with low parental stress (23), where parental stress was assessed by perceived stress scale (PSS >4) (37). These results from the CHS are consistent with a growing body of evidence from international studies indicating that that exposure to vehicle emissions increases the risk of new-onset asthma (4,38,39).
Air pollution effects on children with asthma
Air pollution may play a role in the exacerbation of existing asthma. In the CHS, children with physician-diagnosed asthma had more chronic lower respiratory tract symptoms including bronchitis and phlegm production if they lived in communities with higher levels of NO2, PM10, and PM2.5 (40). Two pollutant models showed that within-community variations in organic carbon (OC) (OR, 1.41 per ppb; 95% CI: 1.12-1.78) and NO2 (OR, 1.07 per ppb; 95% CI: 1.02-1.13) had robust positive associations with the risk of bronchitis symptoms after adjusting for age, maternal and child’s smoking history, sex, race, community and other pollutants including PM, O3, organic and inorganic acid, and elemental carbon (EC) (18).
Air pollution was also associated with acute respiratory symptoms including wheezing and asthma medication use. Amongst fourth-grade school children, an IQR (13.39 μg/m3) increase in monthly average PM10 was associated with almost a 3-fold higher monthly prevalence of wheezing during the spring and summer months after adjusting for age, sex, race/ethnicity, community, home characteristics, and secondhand tobacco smoke (OR, 2.91; 95% CI: 1.46-5.80), but this association was not significant during the fall and winter months (19). Pollutants primarily produced by photochemistry were associated with asthma medication use. IQR increases in monthly average O3 (27.83 ppb), nitric acid (HNO3) (1.64 ppb), and acetic acid (2.66 ppb) levels were associated with 80% (OR, 1.80; 95% CI: 1.19-2.70), 80% (OR, 1.80; 95% CI: 1.23-2.65) and 60% (OR, 1.57; 95% CI: 1.11-2.21) more monthly prevalence of asthma medication use (19). Associations between air pollutants and asthma medication use were stronger among children who spent more time outdoors (OR, 3.07; 95% CI: 1.61-5.86 for O3; OR, 1.93; 95% CI: 1.18-3.15 for HNO3; and OR, 2.38; 95% CI: 1.37-4.14 for acetic acid, respectively), compared to children who spent less time outside. Recent findings suggest that traffic-related pollution was also associated with children’s wheezing (41). Among kindergarten and first grade (Cohort E) children aging 4.4- to 8.9-year-old who were diagnosed with asthma, per increase of 9 minutes in school commuting time was significantly associated with 50% increase (OR, 1.54; 95% CI: 1.01-2.36) of prevalence of severe wheezing using the criteria from the International Study of Asthma and Allergies in Childhood (ISAAC) (42) after adjusting for age, sex, race, community, mode of travel to school, and modeled residential traffic-related pollution. This association was more striking among asthmatic children with commuting times 5 minutes or longer (OR, 1.97; 95% CI: 1.02-3.77). Other effects of air pollution on asthmatic children include increased emergency department visits or hospitalizations (43), and higher school absence rates (44).
Taken together, these results from the CHS demonstrate that the effects of ambient air pollution and traffic-related air pollution on childhood asthma pose a large burden to public health and the economy. According to the CHS estimates, the successful improvement in O3 levels in Southern California during the year 1990 to 1999 reduced more than 2.8 million school absences, which saved more than $220 million (45). On the other hand, asthma burden attributable to air pollution in two California communities was $18 million yearly during 1996 to 2004, and half of this cost was due to traffic-related pollution (46).
Air pollution and lung function
The deficit in the growth of lung function is another chronic health effect of air pollution. Following children from age 10 to 18 years, deficits in the growth of forced expiratory volume in one second (FEV1) were associated with exposure to higher levels of NO2, PM2.5, EC, and acid vapor after adjusting for sex, Hispanic ethnicity, log-transformed height, BMI, BMI squared, present asthma status, child’s smoking history, secondhand tobacco smoke, community, exercise or respiratory tract illness on the day of the test, and indicator variables for field technician (P=0.005, 0.04, 0.007, and 0.004, respectively) (14). Deficits in the growth of forced vital capacity (FVC) were associated with exposure to NO2 and acid vapor (P=0.05 and 0.03, respectively), and deficits in the growth of maximal midexpiratory flow rate (MMEF) were associated with exposure to NO2 and EC (P=0.02 and 0.04, respectively). Similar associations were also observed for FEV1 attained at the age of 18 years (14). For example, the estimated proportion of 18-year-old subjects with a low FEV1 (defined as a ratio of observed to expected FEV1 of less than 80%) in the community with highest level of PM2.5 was 4 times more than the community with the lowest level of PM2.5 (7.9% vs. 1.6%, P=0.002).
Exposures to traffic-related pollution were associated with lung development as well. After adjusting for height, height squared, BMI, BMI squared, present asthma status, community, exercise or respiratory illness on the day of the test, any tobacco smoking by the child in the last year and field technician, children who lived within 500 m of a freeway had significant deficits in FEV1 and MMEF growth from age 10 to 18 compared to children who lived more than 1,500 m from a freeway (P=0.01 and 0.03, respectively) (12). Joint models revealed that adverse effects of traffic exposures on the growth of FEV1 were independent of regional air pollutions (NO2, Acid vapor, PM10, PM2.5, and EC). In another cross-sectional analysis of children with mean age of 11.2 years, residential proximity to a freeway was shown to be inversely associated with the reduction in FVC after adjusting for log-transformed height and height squared, BMI and BMI squared, age, sex, race/ethnicity, community, respiratory illness on the day of the test and field technician (13). Living within 500 m of a freeway was associated with 2% deficit in FVC (P=0.009). Additionally, higher model-based estimate of near-roadway (freeways, highways and large surface streets) NOx was associated with deficits in FEV1 and FVC (P=0.005 and 0.048, respectively) (13). Consistent with our previous findings, near-roadway NOx and regional air pollutants (O3, PM2.5, PM10 and NO2) had independent inverse association with deficits in FEV1 and FVC. There was an evidence that associations between residential near-roadway NOx and deficits in FEV1 and FVC might be modified by parental stress (both interaction P47). Significant inverse associations were only observed among children from high-stress households (parental PSS >4) after adjusting for log height and log height squared, BMI and BMI squared, age, sex, race/ethnicity, community, respiratory illness on the day of the test and field technician, but not among children from low-stress households (parental PSS ≤4). However, no interactions were found for air pollution with sex and asthma status.
Air pollution and airway inflammation
Airway inflammation is a potential mechanism underlying the effects of air pollution on asthma exacerbations (48). The exhaled nitric oxide fraction (FeNO) is a noninvasive marker of aspects of airway inflammation that has been developed and validated in the past decade (49,50). Children with FeNO in the highest quartile at the start of follow-up (>14.8 ppb at 50 mL/s) had more than a 2-fold increased risk of new-onset asthma compared to children with FeNO in the lowest quartile (51). In the CHS, both regional air pollution and traffic-related pollution were associated with higher FeNO. Among children ages 7 to 11 years old, daily 24-h cumulative lagged averages of PM2.5 (over 1-8 days), PM10 (over 1-7 days) and O3 (over 1-23 days) were significantly associated with 17.4% (PNO levels over the IQR (7.5 µg/m3, 12.97 µg/m3, and 15.42 ppb for PM2.5, PM10, and O3, respectively) of each pollutant, respectively, after adjusting for age, sex, race/ethnicity, community, asthma, asthma medication use, history of respiratory allergy, time of FeNO collection, BMI, secondhand tobacco smoke, parental education, language of the questionnaire (English/Spanish), season and whether FeNO testing was conducted outdoors (16). These associations did not significantly vary by sex, asthma, and respiratory allergy status. However, results suggested that the effects of air pollutants were relatively larger in the warm season compared to the cold season. Longitudinal analysis showed increases of long-term (annual average) exposures of NO2 and PM2.5 (scaled to IQR of 1.8 ppb and 2.4 µg/m3, respectively) were associated with 2.29 ppb (P=0.02) and 4.94 ppb (P=0.005) increase in FeNO after adjusting for age, sex, race/ethnicity, asthma, asthma-medication use, history of respiratory allergy, day of FeNO collection, season, and short-term (lags of up to 60 days prior to the day of FeNO test) effects of the same air pollutant (52).
From a set of traffic-related pollution metrics, only the length of road in a circular buffer around the residence was found to be positively associated with FeNO (15). This association was restricted to children with asthma, and was strongest in the 50 m buffer, the smallest buffer considered. Specifically, a 100 m increase in the length of road in a 50 m buffer around subject’s home was associated with a 46.7% (95% CI: 14.3-88.4%) higher FeNO in children with asthma and 0.2% lower (95% CI: −5.5-5.3%) FeNO in children without asthma after adjusting for age, sex, race/ethnicity, community, asthma, asthma-medication use, rhinitis history, BMI percentile, secondhand tobacco smoke, parental education, month and hour of FeNO collection and outdoor testing. Our future work will investigate the longitudinal relationships between traffic-related pollutions and FeNO, as well as whether FeNO influences the relationship between air pollution and asthma incidence.
Genetic susceptibility and gene-environmental interaction
In the past 10 years, the CHS has revealed a great amount of evidence for genetic influence on the association between air pollution and respiratory illness (Table S1). The associated genes include GSTs (encoded by GSTM1, GSTP1, and GSTT1), microsomal epoxide hydrolase (EPHX1), catalase (CAT), myeloperoxidase (MPO), heme oxygenase 1 (HMOX-1), tumor necrosis factor (TNF), arginases (encoded by ARG1 and ARG2 genes), inducible nitric oxide synthase (iNOS, encoded by NOS2), and transforming growth factor β1 (TGFβ1).
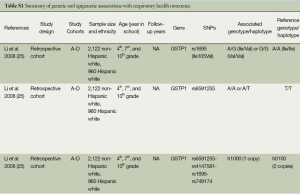
Full table
Incomplete combustions from smoking and fossil fuels contain high levels of polyaromatic hydrocarbons (PAHs), which can lead to oxidative stress and has been shown to relate to asthma and wheeze (53-55). Thus, genes involved in xenobiotic-induced oxidative stress were of great interest. GSTM1 null and GSTP1 (rs1695) A/A genotype were shown to enhance nasal allergic responses with increased IgE levels (56). In the CHS (Table S1), GSTP1 rs1695-G and the upstream promoter single-nucleotide polymorphism (SNP) rs6591255-A allele were both associated with increased occurrences of lifetime asthma and wheezing (25,57). There was a significant interaction between in utero exposure to maternal smoking and rs1695 genotype on the association with wheeze (25). Compared to children with no exposure and rs1695 A/A genotype, children exposed to in utero maternal smoking and having rs1695 A/G or G/G genotypes had a 2-fold increased risk of early-onset asthma, current wheezing and medication use for wheeze after adjusting for age, sex, ethnicity, community, gestational age, and secondhand tobacco smoke (OR, 2.0; 95% CI: 1.1-3.3; OR, 1.9; 95% CI: 1.3-2.6); and OR, 1.9; 95% CI: 1.2-2.8, respectively). In contrast, children carrying rs1695 A/G or G/G genotypes was found to be associated with 40% lower risk of new onset asthma compared to children with rs1695 A/A genotype after adjusting for ethnicity and community (HR, 0.6; 95% CI: 0.4-0.8) (27). The opposite direction of associations between rs1695 genotype with wheezing and asthma incidence suggests rs1695 might have pleiotropic effect on asthma traits.
EPHX1 is also involved in the xenobiotic metabolism, but less studied. We found EPHX1 SNPs rs1051740 and rs2234922 were associated with several asthma outcomes (57) (Table S1). After adjustment for age, sex, race/ethnicity, in utero maternal smoking, number of smokers at home, community, parental education, health insurance and parental history of asthma, children with rs1051740 C/C genotype had a 49% reduced risk of late onset asthma (OR, 0.51; 95% CI: 0.29-0.88) compared to children with T/T genotype. Children with rs2234922 A/G genotype had 42% (OR, 1.42; 95% CI: 1.14-1.76); 45% (OR, 1.45; 95% CI: 1.12-1.89) and 58% (OR, 1.58; 95% CI: 1.19-2.10) increases of lifetime, current and late onset asthma compared to children with A/G genotype. The association between EPHX1 phenotypes and the risk of asthma varied by the GSTP1 rs1695 genotype and residential proximity to a major road. Among children with rs1695 G/G genotype, those had high EPHX1 activity phenotypes were of a 4-fold increased risk of lifetime asthma compared to children with low/intermediate EPHX1 activity phenotypes (OR, 4.0; 95% CI: 1.97-8.16). This association was not significant among children with rs1695 A/A or A/G genotypes. Association between high EPHX1 activity and the increased risk of lifetime asthma was also found among children who lived within 75 m of a major road. Children having high EPHX1 activity phenotype and rs1695 G/G genotype who lived within 75 m of a major road had a 9-fold increased asthma risk compared to those having low/intermediate EPHX1 activity and rs1695 A/A or A/G genotypes, and living more than 75 m of a major road (OR, 8.91; 95% CI: 2.40-33.12). No significant association was found for children living at least 75 m far from a major road.
For lung function, variation in the GST mu family (GSTM2-5) locus was found to be associated with lower FEV1 and MMEF (26) (Table S1). Two haplotypes of GSTM2 (one showed risk effect and one showed protective effect) were significantly associated with 8-year growth of FEV1 and FVC after adjusting for height, height squared, BMI, BMI squared, current asthma status, exercise or respiratory illness on the day of the test, any tobacco smoking by the child in the last year, GSTM1 null genotype, and field technician (all Pin utero maternal smoking. One haplotype of GSTM3 was associated with slower growth of MMEF compared with children with other haplotypes (P=0.002). One haplotype of GSTM4 was associated with decreased growth in FEV1 (P=0.01), FVC (P=0.03), and MMEF (P=0.05) from age 10 to 18. For respiratory illness-related absences, minor alleles in SNPs of GSTP1 including rs6591255-A, rs1695-G, and rs749174-T were associated with a protective effect for respiratory illness-related absences after adjusting for age, sex, race, community, asthma status, family income, health insurance, secondhand tobacco smoke, in utero maternal smoking, and BMI (OR, 0.61; 95% CI: 0.43-0.87 for Hispanic White; and OR, 0.86; 95% CI: 0.71-1.04) for non-Hispanic White) (58). Additionally, the protective effect was restricted among children unexposed to in utero maternal smoking.
Catalase (encoded by CAT), myeloperoxidase (encoded by MPO), and heme oxygenase (encoded by HMOX-1) are enzymes in the oxidative stress defense pathway (59,60). Among children in the CHS (Table S1), we found there was an epistatic interaction of CAT (rs1001179) and MPO (rs2333227) for their association with respiratory-related school absences after adjusting for age, sex, race, community, family income, health insurance, secondhand tobacco smoke, in utero maternal smoking, BMI, cat or dog ownership, and asthma status (61). Children had CAT (rs1001179) G/G genotype and at least one A allele of MPO (rs2333227) had 35% higher risk of respiratory-related school absences compared to children with CAT (rs1001179) G/G genotype and MPO (rs2333227) G/G genotype (OR, 1.35; 95% CI: 1.03-1.77). The epistatic interaction was significant among children living in communities with high O3 level, but was not evident in communities with low O3 level. The number of (GT)n repeats of the HMOX-1 gene showed a bimodal distribution with two peaks being 23 and 30 repeats among Hispanics and non-Hispanic whites (22). Among non-Hispanic whites, children carrying at least one HMOX-1 “short” alleles (22). This association was differentiated by ambient ozone level (interaction P=0.003). Children having at least one “short” allele of HMOX-1 and residing in the low ozone communities had 56% lower risk of asthma incidence than those having no “short” allele of HMOX-1 and living in the low O3 communities (HR, 0.44; 95% CI: 0.23-0.83). No significant association between HMOX-1 and asthma risk was found among Hispanics, suggesting differential asthma risk of this genetic variant by race/ethnicity.
TNF mediates asthma occurrence by initiating airway inflammation and generating airway hyperreactivity (62-64). We previously found DNA sequence variant in rs1800629 modified the association between secondhand smoking and risk of respiratory illness-related school absences (65). In the following work, we found more direct associations between TNF variant and respiratory illness (Table S1). Among children of age 8-11 years old, rs1800629 G/G genotype was associated with 20-30% reduced risk of lifetime asthma (OR, 0.8; 95% CI: 0.7-0.9), life-time (OR, 0.8; 95% CI: 0.7-0.9) and current wheezing (OR, 0.7; 95% CI: 0.6-0.9), and medication for wheezing (OR, 0.7; 95% CI: 0.5-0.8) compared to G/A or A/A genotypes after adjusting for age, sex, race/ethnicity, town, lifetime residence, grade, secondhand tobacco smoke, and in utero maternal smoking (66). The protective effects of the G/G genotype on ever wheezing, current wheezing and medication use for wheeze were two times larger in magnitude for children who lived in low ozone (annual average GSTM1 null compared with the GSTM1 present group. Similarly, the difference in the protective effect of rs1800629 G/G genotype between low and high ozone exposure was larger among children with GSTP1 (rs1695) A/A genotype than children with rs1695 A/G or G/G genotypes. The interaction between the rs1800629 G/G genotype and O3 was also found in the association with bronchitic symptoms among asthmatic children (29). The rs1800629 G/G genotype was associated with 47% reduced risk of bronchitic symptoms for asthmatic children who were exposed to low ambient O3 after adjusting for age, sex, ethnicity, grade, secondhand tobacco smoke, lifetime residence, and community (OR, 0.53; 95% CI: 0.31-0.91). The protective effect was not found among children living in high O3 communities.
Arginases play an important role in asthma pathogenesis through nitrosative stress-mediated airway inflammation (64,67-69). CHS results showed both ARG1 and ARG2 were globally associated with asthma prevalence (28) (Table S1). Compared to the most common ARG1 haplotype that carried the wild-type allele for seven tagged SNPs, one ARG1 haplotype carrying the variant allele (T) for rs2749935 was associated with a 45% reduced risk of asthma after adjusting for age, sex, ethnicity, child’s atopic status, parental history of asthma, parental education, secondhand tobacco smoke, in utero maternal smoking, health insurance, and community (OR, 0.55; 95% CI: 0.36-0.84). Each variant allele (G) of ARG2 SNP rs3742879 was associated with a 31% increase in asthma risk (OR, 1.35; 95% CI: 1.04-1.76). Atopy and ambient O3 modified the association between one ARG1 haplotype and the risk of asthma (interaction P=0.04 and 0.02, respectively). This particular ARG1 haplotype was associated with reduced asthma risk among atopic children or children living in high O3 communities, but was not associated among non-atopic children or children living in communities with low level of O3. No significant interactions were found for ARG2 haplotypes or SNPs with atopy and O3 in the association with asthma risk. In addition to the observed associations for genetic variations of ARG, epigenetic variations in ARG were also investigated for its role in modulating FeNO levels in children. In the CHS, DNA methylation in ARG2 was significantly associated with airway inflammation among children with mean age of 9 years old (70). A 1% increase in average DNA methylation of ARG2 was associated with a 2.3% (95% CI: −4.0% to −0.6%) decrease in FeNO after adjusting for age, sex, race, plate, town, month of DNA collection, asthma medication use, and parental education. This association was more striking among asthmatic children than children without asthma (interaction P=0.01). A similar interaction was also found for ARG1, though little association existed between DNA methylation of ARG1 and FeNO.
Another gene involved in the nitrosative stress is NOS2, which produces NO in response to environmental stimuli (71-74). CHS results showed seven SNPs in the promoter region of NOS2 were globally associated with an increased risk of new-onset asthma (P=0.002) and a lower growth of FEV1 (P=0.02) (75) (Table S1). Further analysis indicated that a pair of “yin-yang” haplotypes of these seven SNPs contributed to the association. One copy of the “yin” haplotype (h0111101) was associated with a 49% increased risk of new-onset asthma compared with children without this haplotype controlling for communities with age- and sex-specific baseline hazard (HR, 1.49; 95% CI: 1.03-2.14), and this association was dose-dependent. In contrast, the “yang” (h1000010) haplotype was associated with 34% (HR, 0.66; 95% CI: 0.49-0.88) reduced risk of new-onset asthma and 48.9 mL (95% CI: 11.6-86.2 mL) higher 8-year FEV1 growth. Interestingly, the increased risk of new-onset asthma for the “yin” haplotype was only found among children who had GSTM1 null genotype (interaction P=0.002). However, the protective effect of the “yang” haplotype did not vary by the GSTM1 genotype. To investigate NOS2 associations with airway inflammations, we found PM2.5, DNA methylation in iNOS were jointly associated with FeNO after adjusting for age, sex, ethnicity, asthma, respiratory allergy, parental education, community, month of FeNO collection, NOS2 promoter haplotypes and experimental plate (76). Among children at the highest 10th percentile of iNOS methylation (>56.6%), higher ambient PM2.5 was associated with higher FeNO (P=0.0002); whereas such an association was not significant among children at lower methylation levels.
Because TGF-β1 is involved in airway inflammation (77,78) and remodeling (79,80), the functional polymorphisms in the TGFB1 gene may play a role in asthma occurrence. We found children with the SNP rs4803457 T/T genotype had a 1.8-fold increased risk of early persistent asthma (asthma as diagnosis before age 3 years with at least one episode of wheeze or asthma medication use after starting first grade) compared to children with C/C or C/T genotypes after adjusting for age, sex, ethnicity, atopic status, parental history of asthma, family income, parental education, in utero maternal smoking, number of smokers at home, insurance, and community (OR, 1.81; 95% CI: 1.11-2.95) (81) (Table S1). This association was varied by the residential proximity to a freeway (interaction P=0.02). The T/T genotype was associated with more than 3-fold increased risk of lifetime asthma among children living within 500 m of a freeway. However, such an association was not significant among children who lived more than 500 m from a freeway. In utero exposure to maternal smoking was previously found to be associated with higher risk of asthma (82). We additionally found such an association can vary by TGFB1 genotypes (interaction P=0.1) (81). The association between in utero exposure to maternal smoking and increased risk of early persistent asthma was only observed among children with T/T genotype (OR, 3.15; 95% CI: 0.81-12.26), but not among children with C/C or C/T genotypes (OR, 0.97; 95% CI: 0.57-1.66).
Discussion
Although air pollution levels have decreased over the last decades (Figure 1), the CHS found both regional and traffic-related pollutants are associated with increased asthma prevalence and new-onset asthma, increased risk of both chronic and acute respiratory symptoms for children with asthma, slower lung function development, and higher airway inflammation. Effects of traffic-related pollutions are independent of effects of regional pollutions. The mechanisms underlying the observed associations may involve multiple genetic influences, gene-environmental interactions, and the interactions between air pollution and other exposures such as in utero maternal smoking and parental stress.
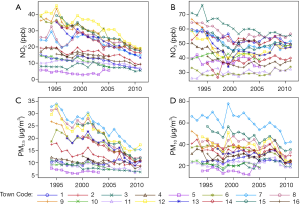
The CHS results provide evidence that air pollution is a major challenge to public health with respect to childhood respiratory illnesses, especially for countries whose air quality is worse than in the United States. Substantial lifelong adverse effects are a real threat if children’s exposures are not reduced. Many approaches can be applied to reduce air pollution exposures including both primary and secondary strategies (30).
“Primary strategies” which reduce the release of air pollutants are critical for the reduction of regional ambient air pollution levels and local traffic-related pollutant levels. Such strategies require the stringent control of automobile and truck emissions. Even under current regulatory levels of air pollutants, adverse effects of air pollution occur for many respiratory illnesses including asthma, low lung function growth, and airway inflammation. These results suggest stricter regulatory standards are needed to prevent adverse health outcomes in the US, Europe and other developed nations. Additional pollutants which are not included in the current standard need to be targeted in the future based on the growing knowledge of their detrimental impact on public health, including ultrafine particles and PAHs. Traffic-related pollutions are major risk factors, but there are no federal regulatory standards for traffic-related pollutions as for regional air pollutions by NAAQS that was put in place as mandated by the 1970s Clean Air Act. Regulation of traffic-related pollutants would be appropriate to protect children’s respiratory health.
Given the limitations in the current regulations and the long time necessary to revise regulations, “secondary strategies” to reduce exposure or to decrease personal susceptibility may also be required. Such strategies could include citing schools and parks away from roads with high traffic volumes; issuing warnings to the public with recommendations for reducing outdoor activity on high pollution days; and minimizing commuting time on roads especially for school commutes.
The strengths of CHS are the long-term, prospective follow-up of five large cohorts of children, with exposure and outcome data collected consistently. We have used central air monitors to measure regional pollution, and different traffic metrics to estimate traffic-related pollutions. Target genes, GWAS, and DNA methylation data are available for assessing the genetic and epigenetic associations with respiratory health outcomes.
However, we acknowledge that some challenges exist for our future studies. First, although different air pollution exposure models such as dispersion model and land use regression models, and air monitors have been used to estimate and measure ambient and traffic-related pollutions, incorporating activity patterns in time and space (home, school, commute, and workplace) (11) in estimating risk estimate remains a challenge particularly for investigating chronic health effects where personal monitoring (especially in children) is infeasible. Second, identification of the factors involved in asthma etiology has remained a big challenge because of complex interplay between environmental and genetic factors. While candidate gene approach has showed promising interactive effects of ambient air and traffic-related pollution on respiratory health, GWAS efforts has not yielded new susceptibility loci for air pollution mediated effects. Additionally, much of the variability of asthma cannot be explained by known asthma-related SNPs. Third, use of epigenetics as a mediating factor of ambient air and traffic-related pollution with health outcomes has received interest in scientific community, but there are challenges ahead with evaluating pollution effects in biological samples with mixed cell populations from surrogate tissues (rather than the tissue of interest, lung or airway in this instance, which is infeasible in children or in population-based study), and that epigenetic variation occurring with short-term exposure making it difficult to use these variations for long-term effects.
In conclusion, air pollution has important adverse effects on respiratory illnesses, which may be mediated in part by genes, tobacco smoke exposures and parental stress. Future research is warranted to better define the long-term effects of air pollution including the relationship between early life exposure to air pollution and health outcomes after into adulthood. Individual interventions based on personal susceptibility may be needed to efficiently prevent adverse effects attributable to air pollution while control measures are being implemented. Lastly, more aggressive air pollution regulations are needed to achieve improved public health benefits for future generations of children.
Acknowledgements
Z.C., M.T.S., and F.D.G. wrote the article. S.P.E. and C.V.B. edited the article and contributed to discussion. All authors reviewed the article. Z.C. and F.D.G. are the guarantors of this work, and as such, take responsibility for the integrity of the data and the accuracy of the data analysis.
This work was supported by the Southern California Environmental Health Sciences Center (grant 5P30ES007048); National Institute of Environmental Health Sciences (grants 5P01ES011627, ES021801, ES023262); and the Hastings Foundation.
Disclosure: The authors declare no conflict of interest.
References
- Health effects of outdoor air pollution. Part 2. Committee of the Environmental and Occupational Health Assembly of the American Thoracic Society. Am J Respir Crit Care Med 1996;153:477-98. [PubMed]
- Brunekreef B, Holgate ST. Air pollution and health. Lancet 2002;360:1233-42. [PubMed]
- McConnell R, Berhane K, Yao L, et al. Traffic, susceptibility, and childhood asthma. Environ Health Perspect 2006;114:766-72. [PubMed]
- Brauer M, Hoek G, Smit HA, et al. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur Respir J 2007;29:879-88. [PubMed]
- Kim JH, Kim JK, Son BK, et al. Effects of air pollutants on childhood asthma. Yonsei Med J 2005;46:239-44. [PubMed]
- Health effects of outdoor air pollution. Committee of the Environmental and Occupational Health Assembly of the American Thoracic Society. Am J Respir Crit Care Med 1996;153:3-50. [PubMed]
- Delfino RJ. Epidemiologic evidence for asthma and exposure to air toxics: linkages between occupational, indoor, and community air pollution research. Environ Health Perspect 2002;110 Suppl 4:573-89. [PubMed]
- Gauderman WJ, Avol E, Lurmann F, et al. Childhood asthma and exposure to traffic and nitrogen dioxide. Epidemiology 2005;16:737-43. [PubMed]
- Jerrett M, Shankardass K, Berhane K, et al. Traffic-related air pollution and asthma onset in children: a prospective cohort study with individual exposure measurement. Environ Health Perspect 2008;116:1433-1438. [PubMed]
- McConnell R, Islam T, Shankardass K, et al. Childhood incident asthma and traffic-related air pollution at home and school. Environ Health Perspect 2010;118:1021-6. [PubMed]
- McConnell R, Berhane K, Gilliland F, et al. Asthma in exercising children exposed to ozone: a cohort study. Lancet 2002;359:386-91. [PubMed]
- Gauderman WJ, Vora H, McConnell R, et al. Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet 2007;369:571-7. [PubMed]
- Urman R, McConnell R, Islam T, et al. Associations of children's lung function with ambient air pollution: joint effects of regional and near-roadway pollutants. Thorax 2014;69:540-7. [PubMed]
- Gauderman WJ, Avol E, Gilliland F, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med 2004;351:1057-67. [PubMed]
- Eckel SP, Berhane K, Salam MT, et al. Residential traffic-related pollution exposures and exhaled nitric oxide in the children's health study. Environ Health Perspect 2011;119:1472-7. [PubMed]
- Berhane K, Zhang Y, Linn WS, et al. The effect of ambient air pollution on exhaled nitric oxide in the Children's Health Study. Eur Respir J 2011;37:1029-36. [PubMed]
- EPA: U.S. Environmental Protection Agency. National Ambient Air Quality Standards (NAAQS). Available online: http://www.epa.gov/air/criteria.html. Accessed on 18 March 2014.
- McConnell R, Berhane K, Gilliland F, et al. Prospective study of air pollution and bronchitic symptoms in children with asthma. Am J Respir Crit Care Med 2003;168:790-7. [PubMed]
- Millstein J, Gilliland F, Berhane K, et al. Effects of ambient air pollutants on asthma medication use and wheezing among fourth-grade school children from 12 Southern California communities enrolled in The Children's Health Study. Arch Environ Health 2004;59:505-14. [PubMed]
- Rondeau V, Berhane K, Thomas DC. A three-level model for binary time-series data: the effects of air pollution on school absences in the Southern California Children's Health Study. Stat Med 2005;24:1103-15. [PubMed]
- Islam T, Gauderman WJ, Berhane K, et al. Relationship between air pollution, lung function and asthma in adolescents. Thorax 2007;62:957-63. [PubMed]
- Islam T, McConnell R, Gauderman WJ, et al. Ozone, oxidant defense genes, and risk of asthma during adolescence. Am J Respir Crit Care Med 2008;177:388-95. [PubMed]
- Shankardass K, McConnell R, Jerrett M, et al. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proc Natl Acad Sci U S A 2009;106:12406-11. [PubMed]
- Gilliland FD, Berhane KT, Li YF, et al. Children's lung function and antioxidant vitamin, fruit, juice, and vegetable intake. Am J Epidemiol 2003;158:576-84. [PubMed]
- Li YF, Gauderman WJ, Conti DV, et al. Glutathione S-transferase P1, maternal smoking, and asthma in children: a haplotype-based analysis. Environ Health Perspect 2008;116:409-15. [PubMed]
- Breton CV, Vora H, Salam MT, et al. Variation in the GST mu locus and tobacco smoke exposure as determinants of childhood lung function. Am J Respir Crit Care Med 2009;179:601-7. [PubMed]
- Islam T, Berhane K, McConnell R, et al. Glutathione-S-transferase (GST) P1, GSTM1, exercise, ozone and asthma incidence in school children. Thorax 2009;64:197-202. [PubMed]
- Salam MT, Islam T, Gauderman WJ, et al. Roles of arginase variants, atopy, and ozone in childhood asthma. J Allergy Clin Immunol 2009;123:596-602. [PubMed]
- Lee YL, McConnell R, Berhane K, et al. Ambient ozone modifies the effect of tumor necrosis factor G-308A on bronchitic symptoms among children with asthma. Allergy 2009;64:1342-8. [PubMed]
- Künzli N, McConnell R, Bates D, et al. Breathless in Los Angeles: the exhausting search for clean air. Am J Public Health 2003;93:1494-9. [PubMed]
- Gilliland FD. Outdoor air pollution, genetic susceptibility, and asthma management: opportunities for intervention to reduce the burden of asthma. Pediatrics 2009;123 Suppl 3:S168-73. [PubMed]
- Linn WS, Berhane KT, Rappaport EB, et al. Relationships of online exhaled, offline exhaled, and ambient nitric oxide in an epidemiologic survey of schoolchildren. J Expo Sci Environ Epidemiol 2009;19:674-81. [PubMed]
- Linn WS, Rappaport EB, Berhane KT, et al. Exhaled nitric oxide in a population-based study of southern California schoolchildren. Respir Res 2009;10:28. [PubMed]
- Benson PE, Pinkerman KO. eds. CALINE4, a dispersion model for predicting air pollution concentration near roadways. State of California, Dept. of Transportation, Division of Engineering Services, Office of Transportation Laboratory, 1984.
- Gilliland FD, Li YF, Dubeau L, et al. Effects of glutathione S-transferase M1, maternal smoking during pregnancy, and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med 2002;166:457-63. [PubMed]
- Huang SL, Su CH, Chang SC. Tumor necrosis factor-alpha gene polymorphism in chronic bronchitis. Am J Respir Crit Care Med 1997;156:1436-9. [PubMed]
- Cohen S, Williams G. Perceived stress in a probability sample of the United States. In: Spacapam S, Oskamp S. eds. The social psychology of health: Claremont Symposium on applied social psychology. Newbury Park, CA: Sage, 1988:31-67.
- Gruzieva O, Bergström A, Hulchiy O, et al. Exposure to air pollution from traffic and childhood asthma until 12 years of age. Epidemiology 2013;24:54-61. [PubMed]
- Zmirou D, Gauvin S, Pin I, et al. Traffic related air pollution and incidence of childhood asthma: results of the Vesta case-control study. J Epidemiol Community Health 2004;58:18-23. [PubMed]
- McConnell R, Berhane K, Gilliland F, et al. Air pollution and bronchitic symptoms in Southern California children with asthma. Environ Health Perspect 1999;107:757-60. [PubMed]
- McConnell R, Liu F, Wu J, et al. Asthma and school commuting time. J Occup Environ Med 2010;52:827-8. [PubMed]
- Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 1995;8:483-91. [PubMed]
- Meng YY, Rull RP, Wilhelm M, et al. Living near heavy traffic increases asthma severity. Policy Brief UCLA Cent Health Policy Res 2006;1-5. [PubMed]
- Gilliland FD, Berhane K, Rappaport EB, et al. The effects of ambient air pollution on school absenteeism due to respiratory illnesses. Epidemiology 2001;12:43-54. [PubMed]
- Hall JV, Brajer V, Lurmann FW. Economic Valuation of Ozone-Related School Absences in the South Coast Air Basin of California. Contemporary Economic Policy 2003;21:407-17.
- Brandt SJ, Perez L, Künzli N, et al. Costs of childhood asthma due to traffic-related pollution in two California communities. Eur Respir J 2012;40:363-70. [PubMed]
- Islam T, Urman R, Gauderman WJ, et al. Parental stress increases the detrimental effect of traffic exposure on children's lung function. Am J Respir Crit Care Med 2011;184:822-7. [PubMed]
- Peden DB. Air pollution in asthma: effect of pollutants on airway inflammation. Ann Allergy Asthma Immunol 2001;87:12-7. [PubMed]
- Recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med 1999;160:2104-17. [PubMed]
- American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 2005;171:912-30. [PubMed]
- Bastain TM, Islam T, Berhane KT, et al. Exhaled nitric oxide, susceptibility and new-onset asthma in the Children's Health Study. Eur Respir J 2011;37:523-31. [PubMed]
- Berhane K, Zhang Y, Salam MT, et al. Longitudinal effects of air pollution on exhaled nitric oxide: the Children's Health Study. Occup Environ Med 2014;71:507-13. [PubMed]
- Jedrychowski W, Galas A, Pac A, et al. Prenatal ambient air exposure to polycyclic aromatic hydrocarbons and the occurrence of respiratory symptoms over the first year of life. Eur J Epidemiol 2005;20:775-82. [PubMed]
- Miller RL, Garfinkel R, Horton M, et al. Polycyclic aromatic hydrocarbons, environmental tobacco smoke, and respiratory symptoms in an inner-city birth cohort. Chest 2004;126:1071-8. [PubMed]
- Ercan H, Birben E, Dizdar EA, et al. Oxidative stress and genetic and epidemiologic determinants of oxidant injury in childhood asthma. J Allergy Clin Immunol 2006;118:1097-104. [PubMed]
- Gilliland FD, Li YF, Saxon A, et al. Effect of glutathione-S-transferase M1 and P1 genotypes on xenobiotic enhancement of allergic responses: randomised, placebo-controlled crossover study. Lancet 2004;363:119-25. [PubMed]
- Salam MT, Lin PC, Avol EL, et al. Microsomal epoxide hydrolase, glutathione S-transferase P1, traffic and childhood asthma. Thorax 2007;62:1050-7. [PubMed]
- Wenten M, Li YF, Lin PC, et al. In utero smoke exposure, glutathione S-transferase P1 haplotypes, and respiratory illness-related absence among schoolchildren. Pediatrics 2009;123:1344-51. [PubMed]
- Mak JC, Leung HC, Ho SP, et al. Polymorphisms in manganese superoxide dismutase and catalase genes: functional study in Hong Kong Chinese asthma patients. Clin Exp Allergy 2006;36:440-7. [PubMed]
- Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol 2006;533:222-39. [PubMed]
- Wenten M, Gauderman WJ, Berhane K, et al. Functional variants in the catalase and myeloperoxidase genes, ambient air pollution, and respiratory-related school absences: an example of epistasis in gene-environment interactions. Am J Epidemiol 2009;170:1494-501. [PubMed]
- Shah A, Church MK, Holgate ST. Tumour necrosis factor alpha: a potential mediator of asthma. Clin Exp Allergy 1995;25:1038-44. [PubMed]
- Carroll MC, Katzman P, Alicot EM, et al. Linkage map of the human major histocompatibility complex including the tumor necrosis factor genes. Proc Natl Acad Sci U S A 1987;84:8535-9. [PubMed]
- Choi IW. TNF-alpha induces the late-phase airway hyperresponsiveness and airway inflammation through cytosolic phospholipase A(2) activation. J Allergy Clin Immunol 2005;116:537-43. [PubMed]
- Wenten M, Berhane K, Rappaport EB, et al. TNF-308 modifies the effect of second-hand smoke on respiratory illness-related school absences. Am J Respir Crit Care Med 2005;172:1563-8. [PubMed]
- Li YF, Gauderman WJ, Avol E, et al. Associations of tumor necrosis factor G-308A with childhood asthma and wheezing. Am J Respir Crit Care Med 2006;173:970-6. [PubMed]
- Baraldi E, Giordano G, Pasquale MF, et al. 3-Nitrotyrosine, a marker of nitrosative stress, is increased in breath condensate of allergic asthmatic children. Allergy 2006;61:90-6. [PubMed]
- Pijnenburg MW, De Jongste JC. Exhaled nitric oxide in childhood asthma: a review. Clin Exp Allergy 2008;38:246-59. [PubMed]
- Ckless K, van der Vliet A, Janssen-Heininger Y. Oxidative-nitrosative stress and post-translational protein modifications: implications to lung structure-function relations. Arginase modulates NF-kappaB activity via a nitric oxide-dependent mechanism. Am J Respir Cell Mol Biol 2007;36:645-53. [PubMed]
- Breton CV, Byun HM, Wang X, et al. DNA methylation in the arginase-nitric oxide synthase pathway is associated with exhaled nitric oxide in children with asthma. Am J Respir Crit Care Med 2011;184:191-7. [PubMed]
- Ricciardolo FL, Sterk PJ, Gaston B, et al. Nitric oxide in health and disease of the respiratory system. Physiol Rev 2004;84:731-65. [PubMed]
- Ricciardolo FL, Di Stefano A, Sabatini F, et al. Reactive nitrogen species in the respiratory tract. Eur J Pharmacol 2006;533:240-52. [PubMed]
- Gabazza EC, Taguchi O, Tamaki S, et al. Role of nitric oxide in airway remodelling. Clin Sci (Lond) 2000;98:291-4. [PubMed]
- Prado CM, Leick-Maldonado EA, Yano L, et al. Effects of nitric oxide synthases in chronic allergic airway inflammation and remodeling. Am J Respir Cell Mol Biol 2006;35:457-65. [PubMed]
- Islam T, Breton C, Salam MT, et al. Role of inducible nitric oxide synthase in asthma risk and lung function growth during adolescence. Thorax 2010;65:139-45. [PubMed]
- Salam MT, Byun HM, Lurmann F, et al. Genetic and epigenetic variations in inducible nitric oxide synthase promoter, particulate pollution, and exhaled nitric oxide levels in children. J Allergy Clin Immunol 2012;129:232-9. [PubMed]
- de Boer WI, van Schadewijk A, Sont JK, et al. Transforming growth factor beta1 and recruitment of macrophages and mast cells in airways in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;158:1951-7. [PubMed]
- Fong CY, Pang L, Holland E, et al. TGF-beta1 stimulates IL-8 release, COX-2 expression, and PGE(2) release in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2000;279:L201-7. [PubMed]
- Kokturk N, Tatlicioglu T, Memis L, et al. Expression of transforming growth factor beta1 in bronchial biopsies in asthma and COPD. J Asthma 2003;40:887-93. [PubMed]
- Redington AE, Madden J, Frew AJ, et al. Transforming growth factor-beta 1 in asthma. Measurement in bronchoalveolar lavage fluid. Am J Respir Crit Care Med 1997;156:642-7. [PubMed]
- Salam MT, Gauderman WJ, McConnell R, et al. Transforming growth factor- 1 C-509T polymorphism, oxidant stress, and early-onset childhood asthma. Am J Respir Crit Care Med 2007;176:1192-9. [PubMed]
- Li YF, Langholz B, Salam MT, et al. Maternal and grandmaternal smoking patterns are associated with early childhood asthma. Chest 2005;127:1232-41. [PubMed]


