
Non-significant efficacy of icotinib plus pleurodesis in epidermal growth factor receptor positive mutant lung cancer patients after malignant pleural effusion drainage compared to icotinib alone
Introduction
Non-small cell lung cancer (NSCLC) is diagnosed in 80% of patients who present with lung cancer (1,2). Distant metastasis and malignant pleural effusions (MPEs) usually occur at an advanced stage of the disease (3). On the basis of risk stratification and the extent of the malignancy the median survival time has typically been found to be 1 to 12 months (4), with the mean overall survival (OS) time for patients with MPEs only 3 to 4 months (5-7).
The symptoms of patients with MPEs have a profound impact on their quality of life and include coughing, chest distress and gasping (8). To alleviate these symptoms, the optimal therapy for MPEs patients should be minimally invasive, be well tolerated by the patient, affordable, and produce the shortest stay in hospital (9). At present, several approaches are available to minimize these symptoms namely: catheter drainage; repeated needle drainage thoracentesis; thoracoscopy with pleurodesis; chest tube thoracostomy; or indwelling pleural catheter (IPC) and ATS/STS/STR clinical practice options to manage MPEs have been published in 2018 (10). Recently, therapy targeted at epidermal growth factor receptor (EGFR)-sensitive mutations has become a novel and standard strategy to treat EGFR-positive advanced lung cancer. EGFR-specific tyrosine kinase inhibitors (TKI) were the first antitumor drugs developed to treat patients with EGFR gene-sensitive mutations (11-14). Icotinib was reported as a first-line anti-tumor drug to target EGFR and was developed in China (6), with an effective outcome of OS, but there have been a paucity of studies that have focused on a comparison of the outcomes and adverse events associated with icotinib plus pleurodesis in lung cancer patients with MPEs, compared to those who received icotinib therapy alone.
This retrospective study analyzed (I) the clinical efficacy of the two different methods to treat MPEs in patients with advanced lung cancer who received icotinib and (II) the adverse reactions to pleurodesis, in order to provide a more informed treatment strategy for MPEs in the clinic practice.
Methods
Patient baseline information
We first reviewed the medical records of 51 individuals from 230 lung adenocarcinoma patients with MPE who were EGFR mutation positive and treated in our hospital between Jan 1st 2014 and Dec 31th 2016. The eligible criteria were as follows: (I) all patients were histologically or cytologically and by imaging examinations confirmed NSCLC cases; (II) all patients had clinical symptoms such as chest distress and exertional dyspnea; (III) massive MPEs were confirmed by computed tomography (CT) imaging showing a depth of effusion greater than 50 mm or in X-ray images as pleural effusion accounting for more than half of one side of the chest, while all MPEs were also confirmed cytologically (15,16); (IV) the patients in the study had sensitive EGFR gene mutations (19 deletion or exon 21 point mutation); (V) Eastern Cooperative Oncology Group Performance Status score (ECOG PS) of 1–3. All enrolled patients were allocated into 2 groups on basis of the treatment methods adopted for pleural effusion drainage. One group was given pleurodesis and the other group was not. After pleural effusion drainage, all patients orally administered icotinib as first-line or second-line therapy. Our study was approved by the Institutional Review Board of Shanghai Chest Hospital (KS1720), and written informed consent was obtained from all patients prior to participation in our study.
Analysis of EGFR mutations
For genetic testing, patient tissue samples were excised from primary tumors or metastatic sites and embedded in paraffin to facilitate sectioning. DNA was extracted from the samples using an FFPE DNA kit (AmoyDx, Xiamen, China) and the ARMS assay utilized to detect the mutation status of EGFR (EGFR 18-21).
Therapeutic method
Thoracic puncture was performed under the guidance of B-ultrasound. Pleuro-catheter closed thoracic drainage was established, with a central venous catheter for the course of treatment (2–5 days: average time, 3±1.6 days). After confirmation of no obvious effusions in patients by using B-ultrasound and X-ray test, then patients underwent different treatments for drainage. The icotinib plus pleurodesis group was injected with the antitumor drugs. The icotinib group was not administered any drugs after careful drainage of the thoracic cavity. For the icotinib plus pleurodesis group, we only observed whether the patients had symptoms of chest pain, cough, dyspnea, nausea and/or vomiting. Later, they all subsequently received icotinib (125 mg/day, 3 times a day, p.o.) until they developed disease progression or the occurrence of intolerable icotinib side effects.
Tumor response and MPEs response were evaluated by CT every 4–8 weeks according to the RECIST 1.1 guideline. When pleural effusion increased by more than one intercostal space or increased by 25% of the progression time since grouping, it was defined as the time to progress (TTP). During follow-up, we carried out a periodic B-ultrasound and X-ray evaluating icotinib response and hematologic examination, liver and kidney function checks and recorded any adverse reactions produced by the medication including rash, diarrhea, leukopenia and dry skin.
Evaluation at follow-up
Progression free survival (PFS) was determined from the start day of EGFR-TKI therapy to the confirmed day of lesion progression, death, or the last follow-up assessment.
OS was determined from EGFR-TKI therapy initiation until the day of death or the last follow-up. If the actual survival time could not be determined or if there was no disease progression, the patient status was considered to be the last monitored day of survival time and/or contact date. The OS was updated in December 2018.
Therapeutic evaluation including efficacy and adverse reactions
We first analyzed the PFS after icotinib treatment and then the efficacy of local processing of MPEs defined according to WHO guidelines thus: (I) complete remission (CR): absence of pleural fluid re-accumulation for >4 weeks; (II) partial remission (PR): pleural fluid decreased by ≥50% for >4 weeks; (III) stable disease (SD): pleural fluid decreased by ≤50%) or increased ≥25% for more than 4 weeks; (IV) progressive disease (PD): pleural fluid increased by ≥25%.
The following definitions were employed:
The objective response rate (ORR) = (CR + PR)/N ×100%.
The overall control rate (OCR) = (CR + PR + SD)/N ×100%. Adverse reactions were observed in this study and evaluated by normal terminology criteria for adverse reactions (CTCAE version 4).
Statistical analysis
Statistical analyses were conducted using SPSS ver. 20.0. Measurement data when normally distributed are presented as means ± standard deviations and categorical variables as percentages. Non-parametric or t-tests were employed to compare any differences in continuous variables between the two groups. Differences between categorical variables were tested using a χ2 test. Variance analysis was used to analyze potential differences between groups, was conducted using the Kaplan-Meier product method and survival curves were drawn. Differences were taken to be statistically significant when the P value was <0.05.
Results
Baseline characteristics of patients
In the present study, 51 patients with MPEs were recruited, of which 22 were females and 29 males. Fourteen cases were in icotinib plus pleurodesis group and 37 cases in the icotinib group. The average age was 62.4±13.6 years in icotinib plus pleurodesis group and 58.8±13.9 years in the other group, ranged from 30 to 79 years. Age stratification revealed 17 cases aged ≥65 years and 34 cases <65 years. Seventeen patients were smokers and 34 non-smokers. Scores of eastern cooperative oncology group performance status (ECOG) were 1 score (n=16) and 2 score (n=35). Gene detection for EGFR revealed an EGFR mutation in 51 patients (an exon 21 point mutation or an exon 19 deletion). All of the subjects were free of other malignant tumors or were prescribed EGFR-TKIs (e.g., gefitinib, erlotinib) therapy. The functions of major organs in these patients were normal before therapy (data not shown). The 51 patients all had adenocarcinoma and in stage IV of TNM (AJCC seventh edition) were divided into two groups: the icotinib plus pleurodesis group (n=14) and icotinib group (n=37). The baseline data of patients showed no significance differences and the two groups were comparable with respect to gender, average age, ECOG scores, pathological types, the TNM stage based on classification of the AJCC seventh edition (9). EGFR mutations were not significantly different between two groups (P=0.242, Table 1).
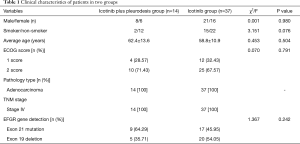
Full table
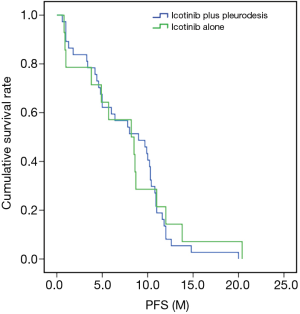
Comparison of PFS in the icotinib plus pleurodesis and icotinib groups
The median PFS of the icotinib plus pleurodesis group was 8.4±1.48 months, while that of the icotinib group was 9.0±0.47 months, with no significant difference (P=0.996, χ2=7.241). The PFS survival curve of the two groups is shown in Figure 1. Date accord with normal distribution by homogeneity test of variances. There were no significant differences in CR PR, SD and PD between the two groups (P=0.993, χ2=0.086). The ORR of the icotinib plus pleurodesis group was 64.29% and that of the other group was 67.57% with no significant difference between the two groups (P=0.824, χ2=0.049, Table 2).

Full table
In addition, the stratified group based on first and second lines of treatment or greater and the outcomes (PFS) of pleurodesis or not for MPEs were analyzed. We found that in the icotinib group, the median PFS of MPEs treated with 2 different lines of treatment showed no statistically significant differences (P=0.816). In the icotinib plus pleurodesis group, there was also no significant difference (P=0.150) in the median PFS of MPEs patients receiving 2 different lines of treatment (Table 3).

Full table
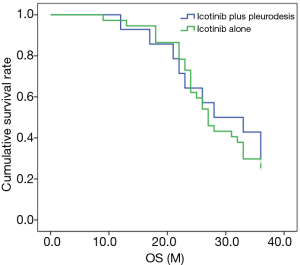
Comparison of OS in the icotinib plus pleurodesis and icotinib groups
As shown in Figure 2, the median OS time of the icotinib plus pleurodesis group was 28.0±6.55 months, while that of the icotinib group was 27.0±1.15 months, with no significant difference (P=0.726, χ2=0.123).
Adverse reactions
Adverse reactions to icotinib for the MPEs patients in the two groups were found in 14 patients on the icotinib plus pleurodesis group, and 37 patients in the icotinib group who did not receive pleurodesis. Of the total 51 patients undergoing icotinib treatment, the adverse reactions of icotinib were: (64.71%: 21 cases of 1 degree, 10 cases of 2 degree, 2 cases of 3 degree) developed a rash; 20 (39.22%: 10 case of 1 degree, 10 cases of 2 degree) complained of diarrhea, 5 (9.80%, 1 degree) nausea and vomiting, 5 (9.80%, 1 degree) developed dry skin and 5 (9.80%, 1 degree) developed leukopenia. Symptoms were relieved after expectant treatments. No one refused icotinib treatment until PD or death. Adverse reactions in the 2 groups were not significantly different. In addition, for the icotinib plus pleurodesis group, adverse reactions of pleurodesis mainly were fever 3 cases (21.43%), chest pain 1 cases (7.14%), gastrointestinal symptom 3 cases (21.43%) and myelosuppression 1 cases (7.14%). The icotinib group did not obviously display these adverse reactions (Table 4).

Full table
Discussion
Previous studies have shown that the EGFR has higher expression levels in many types of tumors and in normal tissues its physiological function is intimately associated with abnormal cell proliferation, neovascularization, distant tumor metastasis and apoptosis (17,18). Published research has also reported that the occurrence of pleural metastasis in lung cancer is closely related to the mutant expression of EGFR. EGFR TKIs selectively inhibit the biological activity of EGFR tyrosine kinases and block signal transduction pathways in abnormal proliferating tumor cells, thereby inhibiting the division and proliferation of tumor cells and thus having significant antitumor activity. This effect can also suppress the production of pleural effusion (12,13,19,20). Icotinib is a first-line, independently developed in China, anticancer drug targeting EGFR (21). The ICOGEN study established the non-inferiority of icotinib when compared with gefitinib, showing that icotinib is a valid therapeutic option for patients with non-small-cell lung cancer as a second-line or third-line treatment. Studies have shown that gefitinib or erlotinib have a better therapeutic effect on lung adenocarcinoma with MPEs, which may be related to the drug concentration achieved in the pleural effusion (22-27). Osimertinib as a 3rd-generation EGFR-TKI is administered as a standard first-line therapy because it improves not only progression-free survival, but OS compared with conventional EGFR-TKIs such as gefitinib. However, EGFR-TKIs including osimertinib have limited efficacy for patients with malignant effusion (28,29). Also in our study, the PFS time of all IV stage patients, whether pleurodesis was performed or not, was only 8.4 and 9.0 months (P=0.996) and also the OS times were not significantly different (P=0.726). The results demonstrated also that the ORRs were 64.29% in the icotinib plus pleurodesis group and 67.57% in the icotinib group without significant difference (P=0.824).
In our study, PFS of lung adenocarcinoma patients with MPEs was not correlated with age, gender or patients being smokers (P=0.504, P=0.980, P=0.076, respectively).
The limitations of the present study were the retrospective design, the small sample number and that only patients treated with icotinib were included.
Conclusions
In summary, after complete drainage of local pleural effusion, EGFR mutant lung adenocarcinoma patients with MPEs may choose to receive targeted therapy without pleurodesis, and can obtain the same therapeutic effect. At the same time, the side reactions caused by pleurodesis are avoided, thereby avoiding impairment the quality of life of patients. However, this study is only a retrospective analysis of a small cohort, which needs to be further supported by the prospective clinical trials of larger numbers of patients. More precise studies are needed, especially if the drugs used in phase IV patients are unified.
Acknowledgments
Funding: This work was supported by the Science and Technology Commission of Shanghai Municipality (grant number 19140902600), Clinical Research Plan of SHDC (grant number 16CR3102B), Collaborative Innovation Center for Translational Medicine at Shanghai Jiao Tong University School of Medicine (grant number TM201610) and Shanghai Chest Hospital Project of Collaborative Innovation (grant numbers YJXT20190210 and YJXT20190210Z), Shanghai Municipal Health Commission Project (grant number 201940221).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.49). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Our study was approved by the Institutional Review Board of Shanghai Chest Hospital (KS1720), and written informed consent was obtained from all patients prior to participation in our study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Powell HA, Tata LJ, Baldwin DR, et al. Treatment decisions and survival for people with small-cell lung cancer. Br J Cancer 2014;110:908-15. [Crossref] [PubMed]
- Chang WJ, Sun JM, Lee JY, et al. A retrospective comparison of adjuvant chemotherapeutic regimens for non-small cell lung cancer (NSCLC): Paclitaxel plus carboplatin versus vinorelbine plus cisplatin. Lung Cancer 2014;84:51-5. [Crossref] [PubMed]
- Morgensztern D, Waqar S, Subramanian J, et al. Prognostic Impact of Malignant Pleural Effusion at Presentation in Patients with Metastatic Non&Small-Cell Lung Cancer. J Thorac Oncol 2012;7:1485-9. [Crossref] [PubMed]
- Clive AO, Kahan BC, Hooper CE, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax 2014;69:1098-104. [Crossref] [PubMed]
- Eleftheriou D, Kallianos A, Adrianopoulos A, et al. AB 28. Evaluation of pleural effusion at first diagnosis of lung cancer. J Thorac Dis 2012. [Crossref]
- Antunes G, Neville E, Duffy J, et al. BTS guidelines for the management of malignant pleural effusions. Thorax 2003;58 Suppl 2:ii29-38. [Crossref] [PubMed]
- Naito T, Satoh H, Ishikawa H, et al. Pleural effusion as a significant prognostic factor in non-small cell lung cancer. Anticancer Res 1997;17:4743-6. [PubMed]
- Bergman B, Aaronson NK, Ahmedzai S, et al. The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer 1994;30a:635-42. [Crossref] [PubMed]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: The time2 randomized controlled trial. JAMA 2012;307:2383-9. [Crossref] [PubMed]
- Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of Malignant Pleural Effusions. An Official ATS/STS/STR Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:839-49. [Crossref] [PubMed]
- Monaco SE, Nikiforova MN, Cieply K, et al. A comparison of EGFR and KRAS status in primary lung carcinoma and matched metastases. Hum Pathol 2010;41:94-102. [Crossref] [PubMed]
- Schmid K, Oehl N, Wrba F, et al. EGFR/KRAS/BRAF mutations in primary lung adenocarcinomas and corresponding locoregional lymph node metastases. Clin Cancer Res 2009;15:4554-60. [Crossref] [PubMed]
- Lieser EAT, Croghan GA, Nevala WK, et al. Up-regulation of pro-angiogenic factors and establishment of tolerance in malignant pleural effusions. Lung Cancer 2013;82:63-8. [Crossref] [PubMed]
- Puri V, Pyrdeck TL, Crabtree TD, et al. Treatment of malignant pleural effusion: a cost-effectiveness analysis. Ann Thorac Surg 2012;94:374-9; discussion 379-80. [Crossref] [PubMed]
- Ryu JS, Ryu HJ, Lee SN, et al. Prognostic impact of minimal pleural effusion in non-small-cell lung cancer. J Clin Oncol 2014;32:960-7. [Crossref] [PubMed]
- Kawamura T, Murakami H, Kobayashi H, et al. Leptomeningeal recurrence after long-term alectinib therapy for non-small cell lung cancer harboring an EML4-ALK fusion protein. Invest New Drugs 2019;37:184-7. [Crossref] [PubMed]
- Liu D, He J, Yuan Z, et al. EGFR expression correlates with decreased disease-free survival in triple-negative breast cancer: a retrospective analysis based on a tissue microarray. Med Oncol 2012;29:401-5. [Crossref] [PubMed]
- Saxena R, Chandra V, Manohar M, et al. Chemotherapeutic Potential of 2-[Piperidinoethoxyphenyl]-3-Phenyl-2H-Benzo(b)pyran in Estrogen Receptor- Negative Breast Cancer Cells: Action via Prevention of EGFR Activation and Combined Inhibition of PI-3-K/Akt/FOXO and MEK/Erk/AP-1 Pathways. PLoS One 2013;8:e66246. [Crossref] [PubMed]
- Hooper CE, Elvers KT, Welsh GI, et al. VEGF and sVEGFR-1 in malignant pleural effusions: association with survival and pleurodesis outcomes. Lung Cancer 2012;77:443-9. [Crossref] [PubMed]
- Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer 2015;88:74-9. [Crossref] [PubMed]
- Masago K, Togashi Y, Fukudo M, et al. Plasma and pleural fluid pharmacokinetics of erlotinib and its active metabolite OSI-420 in patients with non-small-cell lung cancer with pleural effusion. Clin Lung Cancer 2011;12:307-12. [Crossref] [PubMed]
- Jian G, Songwen Z, Ling Z, et al. Prediction of epidermal growth factor receptor mutations in the plasma/pleural effusion to efficacy of gefitinib treatment in advanced non-small cell lung cancer. J Cancer Res Clin Oncol 2010;136:1341-7. [Crossref] [PubMed]
- Kimura H, Fujiwara Y, Sone T, et al. EGFR mutation status in tumour-derived DNA from pleural effusion fluid is a practical basis for predicting the response to gefitinib. Br J Cancer 2006;95:1390-5. [Crossref] [PubMed]
- Junichi S, Shinichi T, Keisuke A, et al. Usefulness of EGFR mutation screening in pleural fluid to predict the clinical outcome of gefitinib treated patients with lung cancer. Int J Cancer 2006;119:2353-8. [Crossref] [PubMed]
- Kubo A, Koh Y, Kawaguchi T, et al. Malignant Pleural Effusion from Lung Adenocarcinoma Treated by Gefitinib. Intern Med 2011;50:745-8. [Crossref] [PubMed]
- Honda Y, Takigawa N, Fushimi S, et al. Disappearance of an activated EGFR mutation after treatment with EGFR tyrosine kinase inhibitors. Lung Cancer 2012;78:121-4. [Crossref] [PubMed]
- Tso‐Fu W, Sung‐Chao C, Jen‐Jyh L, et al. Presence of pleural effusion is associated with a poor prognosis in patients with epidermal growth factor receptor–mutated lung cancer receiving tyrosine kinase inhibitors as first‐line treatment. Asia Pac J Clin Oncol 2017;13:304-13. [Crossref] [PubMed]
- Masuhiro K, Shiroyama T, Suzuki H, et al. Impact of Pleural Effusion on Outcomes of Patients Receiving Osimertinib for NSCLC Harboring EGFR T790M. Anticancer Res 2018;38:3567-71. [Crossref] [PubMed]
- Kawamura T, Kenmotsu H, Kobayashi H, et al. Negative impact of malignant effusion on osimertinib treatment for non-small cell lung cancer harboring EGFR mutation. Invest New Drugs 2020;38:194-201. [Crossref] [PubMed]


