Symptom and functional recovery monitoring in thoracic surgery
Introduction
In recent years, patient-centered care has gained traction as a key component of the quality and value of healthcare. The 2001 Institute of Medicine report on “quality chasm” defines patient-centered care as “providing care that is respectful of, and responsive to, individual patient preferences, needs and values, and ensuring that patient values guide all clinical decisions” (1). Patient-centered care is at the core of a high-quality cancer care delivery system, and includes key attributes such as patient education and empowerment, patient-centered communication, coordination and integration of care, provision of emotional support, access to care, and the involvement of family and friends (2-6).
Patient-reported outcomes (PROs) and patient-generated health data (PGHD) are often used to assess patient-centeredness in healthcare (7), and include measures of symptoms, quality of life (QOL), and functional status using pedometer captured daily steps (8-11). Robust evidence suggests that routine PRO symptom monitoring in cancer care improves patient-provider communication (12-16), and are effective in identifying unrecognized problems (16-20), guiding clinical care (18,21-23), and improving patient outcomes (23-27), including survival (28,29).
Patient-centered care and symptom monitoring are also gaining momentum in thoracic surgery. As surgical mortality has sharply declined in lung cancer and thoracic malignancies, the focus has shifted toward other relevant endpoints, including symptom and functional monitoring (30-33). Symptom and functional monitoring have become increasingly efficient and accurate, due to advances in telemonitoring and wearable technology. The aim of this paper, therefore, is to review the current evidence on symptom and functional monitoring in thoracic surgery, provide an overview of strategies used for monitoring symptoms and functional status, and discuss methods to using PROs and PGHD to drive patient-centered care and clinical decision-making.
Technology-driven strategies for symptom and functional recovery monitoring
Before the advent of digital patient engagement applications, smartphones and other portable electronic devices, symptom assessment in cancer were primarily done either in-person through paper and pencil self-administration of PROs, or via telephone interviews. Interactive, or telephone AVR systems was developed and tested to streamline symptom assessment and management. AVR systems were designed to merge computer software programming with pre-recorded voice within an automated telephone system to assess patient symptoms or health status. They were also capable of incorporating interventions to support patient’s self-management of symptoms and conditions (34,35). Using an AVR system, patients can be contacted at specific times and intervals throughout treatments and after. Patients enter their self-reported symptoms using the telephone touchpad, and their responses are recorded. Clinical staff can use the recorded data to make decisions related to treatments and symptom management.
Evidence suggest that the AVR approach for symptom monitoring have high usability and acceptability. Compared to in-person interviews, AVRs are also more accurate in obtaining patient self-reported symptoms (35,36). Randomized trials show that AVR system-driven symptom monitoring interventions appears equal in efficacy to clinical interviews (35) and promotes self-management skills for symptoms during chemotherapy (34,36). Although AVR systems may be considered inferior to newer modern engagement technologies, the approach may be effective for engaging rural and underserved communities with potential limits to accessing technology or challenges with internet access.
Symptoms can also be monitored electronically via email-based systems and mobile device applications. Email-based systems, such as REDCap, can be used to capture PROs via unique links sent directly to a patient’s email. Data can be stored, in real-time, as patients complete the surveys. PROs can also be built as mobile device applications that can be accessed via smartphones and tablets. Patients can download the application onto their mobile device, and the program can send out reminders directly to the mobile device for patients to complete PROs. Data can be tracked in real-time based on pre-determined thresholds, and graphed to present a specific patient’s symptom trajectory over time. Mobile application approach can be integrated into electronic health records (EHR) and the clinical workflow. Most existing EHR platforms have the capability to collect and integrate PROs [i.e., the Patient-Reported Outcomes Measurement Information System (PROMIS)].
The literature provides some evidence on the expected symptom recovery in lung cancer surgery. The most prevalent symptoms after surgery are pain, fatigue, dyspnea, and coughing (37-39). Postoperatively, symptom intensity generally increases during the first month, and may persist at 3–4 months for select populations. Several predictive factors are associated with symptom intensity, such as type of surgery (open versus minimally invasive), number of comorbid conditions, and persistent tobacco use (37). Dyspnea may persist long-term, with current evidence suggesting that approximately 53% of patients continue to experience breathing challenges 2–3 years after surgery (37). Emotional well-being challenges can also persist postoperatively, with approximately 10% to 25% of patients reporting anxiety symptoms and depressed mood (37).
Objective assessment of functional recovery using accelerometer/pedometer
Due to changes in healthcare and advances in minimally-invasive surgical techniques, patients are discharged earlier after thoracic surgery. In recent years, surgical care is increasingly focused on using Enhanced Recovery after Surgery (ERAS) pathways to improve outcomes (40,41). A typical ERAS pathway may include the following: (I) pre-op food and fluid intake; (II) smoking cessation; (III) pulmonary rehabilitation; (IV) analgesics/pain management; (V) hypothermia precautions; (VI) deep vein thrombosis and antibiotic prophylaxis; (VII) atrial fibrillation prevention; and (VIII) early ambulation (42). A critical gap in ERAS pathways is the lack of remote recovery monitoring capabilities after discharge.
The current delivery model for post-discharge surgical care is largely inefficient and not proactive (43). Patients may contact the hospital when acute problems arise, but are often unaware of when to contact their surgeons. This often requires hours or days to resolve, and is burdensome for surgeons, patients, families, and the healthcare system. Wearable and digital patient engagement technology has the potential to transform the current surgical care paradigm (26,44,45). It has the following advantages compared to current care delivery models: (I) they are highly scalable; (II) they do not depend on a patient’s cognition, language, or health status; (III) they serve as an efficient and unobtrusive method for monitoring postoperative recovery; and (IV) they are deployable in various geographic locations and communities. Importantly, it has the potential to identify patients who are in need of interventions to optimize recovery and outcomes (9,46,47). Our previous research in perioperative telemonitoring demonstrated the feasibility and acceptability of combining PROs, pedometer-tracked daily steps, and feedback/alert system for real-time symptom management based on pre-determined thresholds. We observed a correlation between fewer daily steps and higher risk for postoperative complications (48).
Perioperative symptom and functional recovery monitoring in older adults with cancer
The number of older adults (>65 years) in the U.S. is growing. In 2005 there were 37 million older adults; by 2030 that number will increase to 70 million (49). With a projected 67% increase in cancer incidence by 2030 in adults age 65 and older (50), more robust geriatric surgical oncology data and guidelines are urgently needed.
The physiologic changes of aging, the higher prevalence of comorbidities, and their combined impact on vital organ systems play an important role in an older adult’s ability to tolerate thoracic surgery (51). This could lead to greater risk for adverse postoperative events and prolonged recovery (52,53). For instance, post-operative complications that are more common in older adults have been described as “geriatric events”. These events include (I) failure to thrive and dehydration (81.3%); (II) delirium (17.1%); and (III) mobility-related events—pressure ulcers, falls, and fractures (9.6%) (54). Patients age ≥75 years and those with more comorbidities are more likely to experience geriatric events postoperatively (22.9% to 65.7% higher probability) (54,55). Patients who experienced a geriatric event have a two-fold probability of inpatient complications compared to those with no geriatric events. Geriatric events are also associated with prolonged hospitalizations, higher healthcare costs, higher post-acute care (home health, inpatient rehabilitation facility, skilled nursing facility) and mortality during index hospitalization (54,56).
Comprehensive geriatric assessment has potential to improve the quality of geriatric thoracic surgical oncology care (57). It assesses several patient-centered domains, including: (I) physical functioning; (II) co-morbidities; (III) cognitive function; (IV) psychological well-being; (V) social functioning and support; (VI) medication review; and (VII) nutritional status (58-60). Preoperative geriatric assessment can be used to identify older adults with lung cancer who are at higher risk for suboptimal postoperative outcomes. Geriatric assessment identifies areas of vulnerability and guides targeted interventions to improve postoperative outcomes.
We previously tested a telehealth perioperative physical activity for older adults with lung and GI cancer and their family caregivers (61). Our primary aim was to assess the feasibility and acceptability of the intervention. As part of the intervention, both patients and family caregivers were given a wristband pedometer for self-monitoring daily steps taken throughout the perioperative timeframe. The intervention used comprehensive geriatric assessment and multiple objective functional measures to personalize a walking program for patients, based on their functional capacity and tolerance. Electronic symptom monitoring was also included. Our findings suggest that the intervention was feasible and acceptable to older adults and their family caregivers, and have the potential to promote functional recovery and symptom monitoring in this high-risk population (61,62).
Geriatric assessment domains that are used in surgical oncology include (I) frailty assessment; (II) self-reported unintentional weight loss; (III) performance-based measures of physical function, including gait speed, grip strength assessment using hand dynamometers, and lower extremity functional assessment using the short physical performance battery (SPPB); (IV) history of falls; (V) cognition assessment; (VI) review of comorbidities and medications; and (VII) PROs on physical and psychological symptoms (57,63-67). A recent systematic review concluded that functional status, comorbidity, and frailty were assessed most frequently in the surgical oncology literature, and were most significantly associated with adverse postoperative outcomes (57). Studies suggest that preoperative geriatric assessment predicts the need for discharge to a skilled nursing facility and increased length of hospital stay in surgery (64).
The International Society for Geriatric Oncology (SIOG) Surgical Task Force conducted a multicenter study (PACE) which showed that frailty assessment using the comprehensive geriatric assessment accurately predicted operative mortality and morbidity in older adults with cancer (67). A separate study by task force members found that the Timed Up and Go (TUG) test was predictive of major postoperative complications in older adults who underwent surgery for solid tumor malignancies, including thoracic malignancies (68). Guidelines put forth by the American Geriatrics Society (AGS) and the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) recommend a checklist for optimal preoperative assessment of older adults. The checklist includes screening for (I) cognitive ability; (II) depression; (III) risk factors for delirium; (IV) functional status; (V) history of falls; (VI) nutritional status; (VII) frailty; (VIII) polypharmacy; (IX) treatment goals and expectations; and (X) family/social support system (69,70).
Clinical application of symptom and functional recovery monitoring data
Patient-centered communication and information needs
A key component of quality patient-centered care is communication. Communication in thoracic surgery is often complex and challenging. Thoracic malignancies are complicated diseases with complex multimodal treatments, and patients often have little background to help them understand their diagnosis and treatment options. Epstein and Street propose the following six core functions of patient-centered communication: (I) fostering a healing patient-provider relationship through building rapport and trust; (II) exchanging clinical information and understanding patients’ representations of that information; (III) responding to patients’ emotional needs; (IV) helping patients manage uncertainty; (V) involving patients in the decision-making process; and (VI) enabling patient self-management through supporting patient autonomy and providing appropriate resources. In surgical oncology, these functions are essential to the delivery of quality cancer care (71). The American Society of Clinical Oncology (ASCO) developed consensus guidelines for patient-clinician communication that addresses discussion of goals of care and prognosis, treatment selection, facilitating family involvement in care, and clinician training in communication skills (72).
Thoracic malignancy patients report their treatment-decision making journey as “one of several major healthcare choices” that often began with the decision to undergo surgery, followed by the surgery itself, postoperative recovery, and coping with the consequences (73). Data obtained from symptom and functional monitoring can serve as tools to guide patient-surgeon communication. Communication styles that are direct, realistic, honest, and attentive to the patient’s symptom and functional information needs were the most important factors that contributed to a successful treatment experience (73). Information needs that are ranked as important by patients include (I) preparing for surgery physically and emotionally, (II) coping with pain and other symptoms after surgery, (III) how to care for oneself at home, (IV) managing changes in daily activities and functional status, (V) emotional support, (VI) advance care planning, and (VII) returning to work (74).
Support for family caregivers through remote patient symptom and functional monitoring
Likewise, as patients are discharged from the hospital earlier, a greater proportion of the caregiving burden has fallen on informal/family caregivers. Caregiving in the post-operative setting can be an intense experience with significant impacts on physical and emotional well-being for the family caregiver (75). Family caregivers report unmet needs in all QOL domains. Our own research has found that family caregivers of lung cancer patients experience significant psychological distress, which persists even after patient distress dissipates (76). Major sources of stress include the sense of uncertainty in the patient’s potential for functional decline as well as distress related to managing the patient’s emotional reactions to lung cancer (77). Transitions in care such as from hospital to home recovery are particularly stressful for family caregivers. Often, there is also some disconnect between the patient’s self-perception of health and how family caregivers perceive the patient’s health. Family caregivers often experience increased feelings of powerlessness before and after surgery (75). We are currently studying a self-management intervention to better prepare patients and family caregivers for lung cancer surgery and support them in the recovery period (78). The primary focus of the intervention is to help family caregivers develop self-management skills related to their caregiving role through goal setting, proactive planning, and building problem-solving skills.
Remote patient monitoring tools have the potential to reduce caregiver burden and decrease distress. However, most of these tools have not yet been validated for family caregivers nor have they been widely adopted. The AARP reported in 2016 that 71% of family caregivers are interested in technology to assist with their caregiving, but only 7% were currently using it (79). In a pilot study of a wireless monitoring program before and after major abdominal cancer surgery, we found that wireless monitoring was feasible in the perioperative setting (48). While the pedometer data correlated with PROs, it also revealed some unique information about patients. Whereas PROs returned to baseline at 2 weeks, the number of daily steps was only one-third of the preoperative baseline, perhaps more accurately reflecting functional recovery. In addition, pedometer adherence was significantly higher than survey adherence (88% vs. 65%). Wireless monitoring data also correlated with complications (48).
A model of patient-centered care through symptom and functional monitoring in thoracic surgery
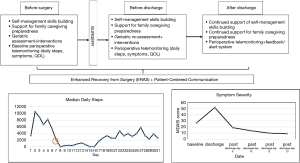
Thoracic surgeons are increasingly asked to provide patient-centered care as a measure of quality and value. Traditional surgical outcomes, such as length of hospital stay, may not accurately reflect the surgical care experience from a thoracic surgery patient’s perspective. Figure 1 presents a potential model of patient-centered care in thoracic surgery that begins before surgery and continues after hospital discharge. The model is based on our previous and ongoing research on patient- and family-centered care in thoracic surgery, and are currently being tested in multiple studies.
Patients can begin using wearable pedometers to monitor baseline functional status and complete baseline electronic PROs for symptom monitoring. This provides a baseline when assessing trajectories of both patient-centered outcomes. Based on the monitoring data, the surgical team can provide quality, timely, and accurate information to patients and families. Families can be given support that promotes their confidence in caregiving before and after surgery. All older adults can complete comprehensive geriatric assessment before surgery; this information can then be used to identify at-risk patients, and develop personalized interventions to reduce geriatric events and poor outcomes. The use of preoperative geriatric assessment is limited in surgical oncology, despite guidelines and recommendations. This could potentially result in frequent under-treatment and over-treatment (80).
During hospitalization, patients can begin wearing pedometers as soon as possible to promote self-management and self-monitoring. Symptoms can be monitored during hospitalization, and appropriate management and information provided to both patient and families. Discharge counseling can be focused on self-management skills building for recovery at home, and continued support for family caregiving preparedness. Discharge planning can include a follow-up geriatric assessment to identify at risk older adults in need of additional support after discharge.
After hospital discharge, continued symptom and functional monitoring can be undertaken for both patients and families. This can be achieved via remote postoperative telemonitoring using ePROs and wearable devices. Patients and families may be most vulnerable during the immediate post-discharge time; symptom and functional monitoring can serve as an efficient and real-time strategy for patient-surgeon communication. A qualitative study on the patient’s experience with a liver surgery ERAS program suggest that a lack of monitoring in the early days of discharge was a major concern for patients (81). Post-discharge telemonitoring can continue with pedometer-captured daily steps, and intermittent ePROs (symptoms, QOL). The data captured via telemonitoring can be graphically-displayed for easy identification of unique trajectories of recovery. A real-time, alert/feedback system based on pre-determined outcome thresholds can be triggered based on the patient’s data. Pre-determined thresholds may include: (I) one or more symptoms rated 4 or greater (moderate-severe intensity); (II) one or more QOL items rated moderate, severe, or unable to accomplish; and (III) daily steps of ≤1,500. When thresholds are met, the thoracic surgery team can receive secure alerts; this will prompt the team to initiate contact with patients via secure messaging as a first attempt to assess and remedy the problem. Telephone calls can be used to further asses, triage, manage, and resolve the issue.
Conclusions
Patient-centered care in thoracic surgery is an essential component of quality surgical oncology care. Routine symptom and functional recovery monitoring using wearable technologies is an evidence-based approach that embodies the very essence of patient-centeredness in healthcare. Nearly two decades after the “quality chasm” report, the consistent delivery of patient-centered cancer care remains a challenge. Through synthesis of strategies for symptom and functional monitoring, we propose that patient-centered care is feasible to implement. Further research is needed to test models of cancer care delivery in thoracic surgery that meets the patient’s and family’s needs, preferences, and values.
Acknowledgments
Funding: Research reported in this paper is supported by the National Cancer Institute of the National Institutes of Health under award number R01CA217841.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Peter J. Kneuertz) for the series “Patient reported Outcomes in Thoracic Surgery: A new Frontier” published in Journal of Thoracic Disease. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.59). The series “Patient reported Outcomes in Thoracic Surgery: A new Frontier” was commissioned by the editorial office without any funding or sponsorship. JK serves as the unpaid editorial board member of Journal of Thoracic Disease from May 2019 to Apr 2021. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclaimer: The statements presented in this article are solely the responsibility of the author(s) and do not necessarily represent the official views of the National Institutes of Health.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the quality chasm: a new health system for the 21st century. Washington DC: National Academies Press (US), 2001.
- Levit L, Balogh E, Nass S, et al. editors. Delivering high-quality cancer care: charting a new course for a system in crisis. Washington DC: National Academies Press (US), 2013.
- Epstein RM, Fiscella K, Lesser CS, et al. Why the nation needs a policy push on patient-centered health care. Health Aff (Millwood) 2010;29:1489-95. [Crossref] [PubMed]
- Bechtel C, Ness DL. If you build it, will they come? Designing truly patient-centered health care. Health Aff (Millwood) 2010;29:914-20. [Crossref] [PubMed]
- Picker Institute. Principles of patient-centered care. 2013. Available online: http://pickerinstitute.org/about/picker-principles
- Epstein RM, Street RL Jr. The values and value of patient-centered care. Ann Fam Med 2011;9:100-3. [Crossref] [PubMed]
- Birkmeyer JD, Dimick JB, Birkmeyer NJ. Measuring the quality of surgical care: structure, process, or outcomes? J Am Coll Surg 2004;198:626-32. [Crossref] [PubMed]
- Chen J, Ou L, Hollis SJ. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res 2013;13:211. [Crossref] [PubMed]
- Beg MS, Gupta A, Stewart T, et al. Promise of wearable physical activity monitors in oncology practice. J Oncol Pract 2017;13:82-9. [Crossref] [PubMed]
- Chung AE, Basch EM. Potential and challenges of patient-generated health data for high-quality cancer care. J Oncol Pract 2015;11:195-7. [Crossref] [PubMed]
- Chung AE, Jensen RE, Basch EM. Leveraging emerging technologies and the "internet of things" to improve the quality of cancer care. J Oncol Pract 2016;12:863-6. [Crossref] [PubMed]
- Jones JB, Snyder CF, Wu AW. Issues in the design of Internet-based systems for collecting patient-reported outcomes. Qual Life Res 2007;16:1407-17. [Crossref] [PubMed]
- Taenzer P, Bultz BD, Carlson LE, et al. Impact of computerized quality of life screening on physician behaviour and patient satisfaction in lung cancer outpatients. Psychooncology 2000;9:203-13. [Crossref] [PubMed]
- Rosenbloom SK, Victorson DE, Hahn EA, et al. Assessment is not enough: a randomized controlled trial of the effects of HRQL assessment on quality of life and satisfaction in oncology clinical practice. Psychooncology 2007;16:1069-79. [Crossref] [PubMed]
- Boyes A, Newell S, Girgis A, et al. Does routine assessment and real-time feedback improve cancer patients' psychosocial well-being? Eur J Cancer Care (Engl) 2006;15:163-71. [Crossref] [PubMed]
- Velikova G, Booth L, Smith AB, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol 2004;22:714-24. [Crossref] [PubMed]
- Mooney KH, Beck SL, Friedman RH, et al. Telephone-linked care for cancer symptom monitoring: a pilot study. Cancer Pract 2002;10:147-54. [Crossref] [PubMed]
- Cleeland CS, Wang XS, Shi Q, et al. Automated symptom alerts reduce postoperative symptom severity after cancer surgery: a randomized controlled clinical trial. J Clin Oncol 2011;29:994-1000. [Crossref] [PubMed]
- Basch E, Artz D, Dulko D, et al. Patient online self-reporting of toxicity symptoms during chemotherapy. J Clin Oncol 2005;23:3552-61. [Crossref] [PubMed]
- Weaver A, Young AM, Rowntree J, et al. Application of mobile phone technology for managing chemotherapy-associated side-effects. Ann Oncol 2007;18:1887-92. [Crossref] [PubMed]
- Butt Z, Wagner LI, Beaumont JL, et al. Longitudinal screening and management of fatigue, pain, and emotional distress associated with cancer therapy. Support Care Cancer 2008;16:151-9. [Crossref] [PubMed]
- Kearney N, McCann L, Norrie J, et al. Evaluation of a mobile phone-based, advanced symptom management system (ASyMS) in the management of chemotherapy-related toxicity. Support Care Cancer 2009;17:437-44. [Crossref] [PubMed]
- Bainbridge D, Seow H, Sussman J, et al. Multidisciplinary health care professionals' perceptions of the use and utility of a symptom assessment system for oncology patients. J Oncol Pract 2011;7:19-23. [Crossref] [PubMed]
- Basch E, Snyder C, McNiff K, et al. Patient-reported outcome performance measures in oncology. J Oncol Pract 2014;10:209-11. [Crossref] [PubMed]
- Bennett AV, Jensen RE, Basch E. Electronic patient-reported outcome systems in oncology clinical practice. CA Cancer J Clin 2012;62:337-47. [Crossref] [PubMed]
- Strasser F, Blum D, von Moos R, et al. The effect of real-time electronic monitoring of patient-reported symptoms and clinical syndromes in outpatient workflow of medical oncologists: E-MOSAIC, a multicenter cluster-randomized phase III study (SAKK 95/06). Ann Oncol 2016;27:324-32. [Crossref] [PubMed]
- Nekhlyudov L, Levit L, Hurria A, et al. Patient-centered, evidence-based, and cost-conscious cancer care across the continuum: Translating the Institute of Medicine report into clinical practice. CA Cancer J Clin 2014;64:408-21. [Crossref] [PubMed]
- Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 2016;34:557-65. [Crossref] [PubMed]
- Denis F, Basch EM, Lethrosne C, et al. Randomized trial comparing a web-mediated follow-up via patient-reported outcomes (PRO) vs. routine surveillance in lung cancer patients: final results. J Clin Oncol 2018;36:6500. [Crossref]
- Brennan MF, Radzyner M, Rubin DM. Outcome--more than just operative mortality. J Surg Oncol 2009;99:470-7. [Crossref] [PubMed]
- Fagundes CP, Shi Q, Vaporciyan AA, et al. Symptom recovery after thoracic surgery: measuring patient-reported outcomes with the MD Anderson Symptom Inventory. J Thorac Cardiovasc Surg 2015;150:613-9.e2. [Crossref] [PubMed]
- Khullar OV, Rajaei MH, Force SD, et al. Pilot study to integrate patient reported outcomes after lung cancer operations into the society of thoracic surgeons database. Ann Thorac Surg 2017;104:245-53. [Crossref] [PubMed]
- Shi Q, Wang XS, Vaporciyan AA, et al. Patient-reported symptom interference as a measure of postsurgery functional recovery in lung cancer. J Pain Symptom Manage 2016;52:822-31. [Crossref] [PubMed]
- Mooney KH, Beck SL, Wong B, et al. Automated home monitoring and management of patient-reported symptoms during chemotherapy: results of the symptom care at home RCT. Cancer Med 2017;6:537-46. [Crossref] [PubMed]
- Sikorskii A, Given CW, Given B, et al. Differential symptom reporting by mode of administration of the assessment: automated voice response system versus a live telephone interview. Med Care 2009;47:866-74. [Crossref] [PubMed]
- Mooney KH, Beck SL, Friedman RH, et al. Automated monitoring of symptoms during ambulatory chemotherapy and oncology providers' use of the information: a randomized controlled clinical trial. Support Care Cancer 2014;22:2343-50. [Crossref] [PubMed]
- Poghosyan H, Sheldon LK, Leveille SG, et al. Health-related quality of life after surgical treatment in patients with non-small cell lung cancer: a systematic review. Lung Cancer 2013;81:11-26. [Crossref] [PubMed]
- Antonoff MB, Ragalie W, Correa AM, et al. Results of postdischarge nursing telephone assessments: persistent symptoms common among pulmonary resection patients. Ann Thorac Surg 2016;102:276-81. [Crossref] [PubMed]
- Dai W, Zhang Y, Feng W, et al. Using patient-reported outcomes to manage postoperative symptoms in patients with lung cancer: protocol for a multicentre, randomised controlled trial. BMJ Open 2019;9:e030041. [Crossref] [PubMed]
- Medbery RL, Fernandez FG, Khullar OV. ERAS and patient reported outcomes in thoracic surgery: a review of current data. J Thorac Dis 2019;11:S976-86. [Crossref] [PubMed]
- Comacchio GM, Monaci N, Verderi E, et al. Enhanced recovery after elective surgery for lung cancer patients: analysis of current pathways and perspectives. J Thorac Dis 2019;11:S515-22. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Smith AB, Basch E. Role of Patient-reported outcomes in postsurgical monitoring in oncology. J Oncol Pract 2017;13:535-8. [Crossref] [PubMed]
- Stover AM, Basch EM. Using patient-reported outcome measures as quality indicators in routine cancer care. Cancer 2016;122:355-7. [Crossref] [PubMed]
- Smith TG, Castro KM, Troeschel AN, et al. The rationale for patient-reported outcomes surveillance in cancer and a reproducible method for achieving it. Cancer 2016;122:344-51. [Crossref] [PubMed]
- Wood WA, Basch E. From intuition to execution: realizing the potential of wearables in oncology. J Oncol Pract 2017;13:90-2. [Crossref] [PubMed]
- Bennett AV, Reeve BB, Basch EM, et al. Evaluation of pedometry as a patient-centered outcome in patients undergoing hematopoietic cell transplant (HCT): a comparison of pedometry and patient reports of symptoms, health, and quality of life. Qual Life Res 2016;25:535-46. [Crossref] [PubMed]
- Sun V, Dumitra S, Ruel N, et al. Wireless Monitoring Program of Patient-Centered Outcomes and Recovery Before and After Major Abdominal Cancer Surgery. JAMA Surg 2017;152:852-9. [Crossref] [PubMed]
- Ries LAG, Melbert D, Krapcho M, et al. SEER cancer statistics review, 1975-2005. Bethesda: National cancer institute, 2008.
- Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 2009;27:2758-65. [Crossref] [PubMed]
- Tufo A, Dunne DF, Manu N, et al. Changing outlook for colorectal liver metastasis resection in the elderly. Eur J Surg Oncol 2019;45:635-43. [Crossref] [PubMed]
- Moorthy K, Wynter-Blyth V. Prehabilitation in perioperative care. Br J Surg 2017;104:802-3. [Crossref] [PubMed]
- Berian JR, Mohanty S, Ko CY, et al. Association of loss of independence with readmission and death after discharge in older patients after surgical procedures. JAMA Surg 2016;151:e161689. [Crossref] [PubMed]
- Tan HJ, Saliba D, Kwan L, et al. Burden of geriatric events among older adults undergoing major cancer surgery. J Clin Oncol 2016;34:1231-8. [Crossref] [PubMed]
- Huisman MG, Audisio RA, Ugolini G, et al. Screening for predictors of adverse outcome in onco-geriatric surgical patients: a multicenter prospective cohort study. Eur J Surg Oncol 2015;41:844-51. [Crossref] [PubMed]
- Sacks GD, Lawson EH, Dawes AJ, et al. Which patients require more care after hospital discharge? An analysis of post-acute care use among elderly patients undergoing elective surgery. J Am Coll Surg 2015;220:1113-21.e2. [Crossref] [PubMed]
- Huisman MG, Kok M, de Bock GH, et al. Delivering tailored surgery to older cancer patients: preoperative geriatric assessment domains and screening tools - a systematic review of systematic reviews. Eur J Surg Oncol 2017;43:1-14. [Crossref] [PubMed]
- Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 2011;29:3457-65. [Crossref] [PubMed]
- Hurria A, Cirrincione CT, Muss HB, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol 2011;29:1290-6. [Crossref] [PubMed]
- Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer 2005;104:1998-2005. [Crossref] [PubMed]
- Lafaro KJ, Raz DJ, Kim JY, et al. Pilot study of a telehealth perioperative physical activity intervention for older adults with cancer and their caregivers. Support Care Cancer 2020;28:3867-76. [Crossref] [PubMed]
- Sun V, Raz DJ, Kim JY, et al. Barriers and facilitators of adherence to a perioperative physical activity intervention for older adults with cancer and their family caregivers. J Geriatr Oncol 2020;11:256-62. [Crossref] [PubMed]
- Dale W, Hemmerich J, Kamm A, et al. Geriatric assessment improves prediction of surgical outcomes in older adults undergoing pancreaticoduodenectomy: a prospective cohort study. Ann Surg 2014;259:960-5. [Crossref] [PubMed]
- Badgwell B, Stanley J, Chang GJ, et al. Comprehensive geriatric assessment of risk factors associated with adverse outcomes and resource utilization in cancer patients undergoing abdominal surgery. J Surg Oncol 2013;108:182-6. [Crossref] [PubMed]
- Ommundsen N, Wyller TB, Nesbakken A, et al. Preoperative geriatric assessment and tailored interventions in frail older patients with colorectal cancer: a randomized controlled trial. Colorectal Dis 2018;20:16-25. [Crossref] [PubMed]
- Robinson TN, Eiseman B, Wallace JI, et al. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg 2009;250:449-55. [PubMed]
- PACE participants, Audisio RA, Pope D, et al. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hematol 2008;65:156-63.
- Huisman MG, van Leeuwen BL, Ugolini G, et al. "Timed Up & Go": a screening tool for predicting 30-day morbidity in onco-geriatric surgical patients? A multicenter cohort study. PLoS One 2014;9:e86863. [Crossref] [PubMed]
- Chow WB, Rosenthal RA, Merkow RP, et al. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg 2012;215:453-66. [Crossref] [PubMed]
- Mohanty S, Rosenthal RA, Russell MM, et al. Optimal perioperative management of the geriatric patient: a best practices guideline from the American College of Surgeons NSQIP and the American Geriatrics Society. J Am Coll Surg 2016;222:930-47. [Crossref] [PubMed]
- Epstein RM, Street Jr RL. Patient-centered communication in cancer care: promoting healing and reducing suffering. Betheda: National Cancer Institute, 2007.
- Gilligan T, Coyle N, Frankel RM, et al. Patient-clinician communication: American Society of Clinical Oncology Consensus Guideline. J Clin Oncol 2017;35:3618-32. [Crossref] [PubMed]
- McCahill LE, Hamel-Bissell BP. The patient lived experience for surgical treatment of colorectal liver metastases: a phenomenological study. Palliat Support Care 2009;7:65-73. [Crossref] [PubMed]
- Gillespie J, Kacikanis A, Nyhof-Young J, et al. Information needs of hepato-pancreato-biliary surgical oncology patients. J Cancer Educ 2017;32:589-95. [Crossref] [PubMed]
- Fujinami R, Sun V, Zachariah F, et al. Family caregivers' distress levels related to quality of life, burden, and preparedness. Psychooncology 2015;24:54-62. [Crossref] [PubMed]
- Kim JY, Sun V, Raz DJ, et al. The impact of lung cancer surgery on quality of life trajectories in patients and family caregivers. Lung Cancer 2016;101:35-9. [Crossref] [PubMed]
- Mosher CE, Jaynes HA, Hanna N, et al. Distressed family caregivers of lung cancer patients: an examination of psychosocial and practical challenges. Support Care Cancer 2013;21:431-7. [Crossref] [PubMed]
- Sun V, Raz DJ, Erhunmwunsee L, et al. Improving family caregiver and patient outcomes in lung cancer surgery: study protocol for a randomized trial of the multimedia self-management (MSM) intervention. Contemp Clin Trials 2019;83:88-96. [Crossref] [PubMed]
- Chi NC, Demiris G. The roles of telehealth tools in supporting family caregivers: current evidence, opportunities, and limitations. J Gerontol Nurs 2017;43:3-5. [Crossref] [PubMed]
- Ghignone F, van Leeuwen BL, Montroni I, et al. The assessment and management of older cancer patients: a SIOG surgical task force survey on surgeons' attitudes. Eur J Surg Oncol 2016;42:297-302. [Crossref] [PubMed]
- Vandrevala T, Senior V, Spring L, et al. 'Am I really ready to go home?': a qualitative study of patients' experience of early discharge following an enhanced recovery programme for liver resection surgery. Support Care Cancer 2016;24:3447-54. [Crossref] [PubMed]