Imaging patterns of early stage lung cancer for the thoracic surgeon
Introduction
In the modern era, thoracic surgeons are experiencing an increase interest in imaging patterns of early stage lung cancer mainly due to the recent introduction of the ground glass opacity (GGO) in clinical practice, and secondly for the necessity to an accurate cancer localization to schedule the appropriate type of resection such as segmentectomy for management of small peripheral lung tumors.
Moreover, the wide adoption of low dose CT-scan made possible the increase diagnosis of GGO. The incidence of malignant component within a GGO can be as high as 63% (1). Although peripheral GGO can be treated with wedge resection or segmentectomy, centrally located GGO lesions are more difficult to resect and may in rare cases require a diagnostic lobectomy.
In general terms, there are three main radiological approaches to diagnose early lung cancer. These include chest radiography, chest tomography and positron emission tomography with their recent improvements such as 3D reconstruction and printing.
In this brief review we will analyze the latest news regarding imaging patterns of pulmonary nodules with special emphasis to ground glass opacity.
Definition of early lung cancer
According to the TNM classification, early stage lung cancer include the 1st and 2nd stage and therefore a tumor of 5 cm with no nodes or distant metastasis is still be regarded as early stage lung cancer; in this paper we define early lung cancer (ELC) a tumor in the lung with a maximum diameter of 2 cm.
Definition of ground glass opacity
Ground glass opacity is an opacity that does not obscure the lung structures such as bronchi and vessels at high resolution CT-scan. A pure type can be distinguished from a mixed one. It can be further sub-classified pulmonary as pure, heterogeneous and part-solid. The first one has no central scar, the heterogeneous has consolidated component visible on parenchyma window. Finally, the part-solid type is visible also on mediastinal window.
Chest radiography
In the real world, it is known that chest X-ray failed to detect 77% of lung cancers, including 79% of tumors smaller than 20 mm and 50% larger than 20 mm (2).
Nevertheless, a recent study on a cohort of 63,228 patients who underwent chest radiography screening demonstrated 38% of ELC in patients receiving chest-X-ray while only 26% were diagnosed without surveillance. Chest radiography surveillance was a factor for mortality reduction in female of 10% at the 3- and 5-year survival time-points (P<0.001). The Authors concluded that chest-X-ray intervals of less than 3 years may detect ELC in female patients (3).
Chest tomography
For the management of pulmonary nodules the American College of Radiology (ACR) stated that thin-slice computed tomography (CT) should be the first choice (4). Furthermore the introduction of low dose CT screening programs for lung cancer is being implemented in the USA, China and Europe (1-3) and certainly will increase the diagnosis of early lung cancer (ELC) including the GGO. Lung cancer screening with low-dose CT has proven to decrease overall and lung cancer specific mortality (2-5). More in detail, in a previous study, lung cancer specificity mortality was 346 versus 425 respectively comparing low-dose CT scan group versus the chest X-ray group; the absolute risk reduction (ARR) was equal to 0.3125%, and the relative risk reduction (RRR) was of 20%. These results suggest a significant reduction of risk (6), with rate of positive screening test equal to 24.2% using low-dose CT and 6.9% with chest radiography over all three rounds of the NLST study (6).
The nodule’s appearance represents a very important factor in the assessment of malignancy using low-dose computed tomography. As reported in previous study (7-9), growth rate is different for solid and subsolid nodules, and sub-solid nodules have higher probability of malignancy: more in detail, in a lung cancer screening study, it has been reported that the prevalence of malignancy was 59% for non-solid nodules, 48% for part-solid nodules and 11% for solid nodules (7-9). In the Early Lung Cancer Action Project, 34% (15/44) of subsolid nodules detected at baseline were malignant: more in detail, for part-solid and non-solid nodules malignant rates were 63% (10/16) and 18% (5/28), respectively; contrary, only 7% of solid nodules were malignant at baseline (7,10).
The new appearance or the enlargement of the solid component within a GGO is suggestive of malignant transformation (11). Furthermore, during follow up stable lesions are often interstitial fibrosis or atypical adenomatous hyperplasia. In a previous study by Kim et al. (12), persistent ground-glass nodules at thin-section CT were histologically assessed; in a total of 53 ground-glass nodules, 36 cases were related to the former bronco-alveolar carcinoma (75%) and 6 cases were related to adenocarcinoma (6%). In the remaining cases, 3 nodules were represented by atypical adenomatous hyperplasia and 10 cases were histologically associated with focal interstitial fibrosis or organizing pneumonia. In conclusion, Kim et al. reported that “these nodules do not manifest morphologic features that distinguish them from other GGO nodules with different histopathologic diagnoses” (12).
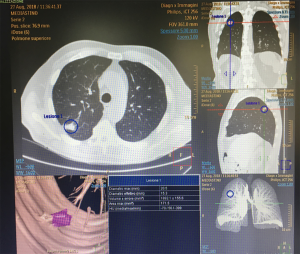
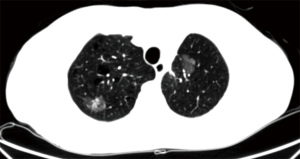
In view of these considerations, Authors have suggested to focus not on morphological features but on growth rate and doubling time at follow-up CT. Hasegawa et al. (13) described that the mean doubling volume time was 813 days, 457 and 149 in pure GGOs, in lesions having ground-glass appearance with central solid component and in solid nodules respectively. Most tumours (80%) were adenocarcinomas; 78% of these have shown pure ground-glass appearance or ground-glass with solid central component. The Authors found a significant difference (P<0.05) for the doubling-time of the three categories of nodular lesions; non-solid lesions seem to growth slowly than solid ones, and this fact has important implication in the management of nodular lesions (13). However, the annual CT screening allowed to detect “slowly growing adenocarcinomas that were not visible on chest radiographs” (13). Certainly the presence of two GGOs in different lungs create more difficulties and thinking (Figure 1). Lee et al. confirmed in 100% of cases that the presence of GGO in a patient with adenocarcinoma is due to the intra-alveolar lepidic growth (14).
3D reconstruction and lung modeling
The three-dimensional (3D) reconstruction produced from multi-detector computed tomography (MDCT) permits surgeons to preoperatively distinguish with precision the anatomic structures of every patient. It appears also evident that 3D imaging reproduces the tumors more precisely than two-dimensional (2D) conventional CT imaging.
Preoperative surgical scheduling with interactive 3D CT reconstruction is certainly a useful method to improve the surgeon’s awareness of the patient’s lung anatomy with its variations. During VATS 3D reconstruction with 3D vision is achievable and could improve the precision of anatomic resection (14-16).
Recently, authors tested in a pilot study the automated segmentation of the lung parenchyma, allowing 3D computed tomography analytic software package assessment of cancer size, location, and assessments of surgical boundaries. The 3D computed tomography analysis showed a positive predictive value of 87% in estimating a marginal clearance >1 cm. The authors concluded that preoperative 3D computed tomography analysis of segmental anatomy confirm the location of a tumor within an anatomic segment and support in predicting surgical margins, and therefore it may assist in the evaluation regarding the appropriateness of segmentectomy for peripheral lung tumors (17).
Another interesting study was performed in 201 patients with stage 1 lung cancer in contact with visceral pleura. Thirty per cent (61 out 201 patients) had pathologically verified visceral pleural invasion. Logistic model analysis showed that the 3D pleural pattern was the only significant factor (P<0.001), and the accuracy of 77% was obtained with a cut-off value of 13.4. The authors concluded that computer-aided 3D CT analysis of the pleura was useful for predicting pleural invasion (18,19). Our experience is similar with the authors, and Figure 2 well demonstrates a small peripheral tumor with pleural invasion which was confirmed at operation.
Furthermore 3D printing could be very useful in patients with lung cancer with variant anatomy of the bronchi. This variation was evaluated by 3D multi-detector CT-angiography with bronchography and a 3D printing model. The printing model was useful for identifying and evaluating the variant bronchi (20). In our experience we use since many years the 3D reconstruction also for major extended operation.
Positron emission tomography
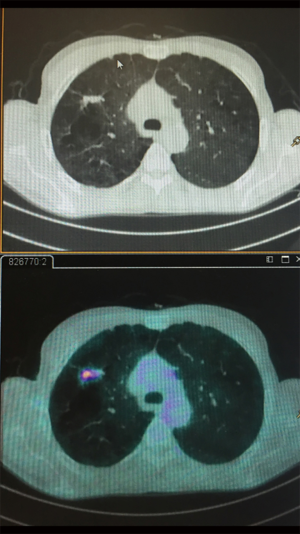
Although the role of FDG-PET imaging in thoracic oncology is well established, and although it has a good sensitivity (96,8%) and specificity (77.8%) in distinguishing cancer in solid pulmonary nodules, the sensitivity in assessing sub-solid lesions is much lower (50%); lepidic carcinomas, which typically appear as GGO on CT, are FDG-PET negative. Therefore the adoption of PET-CT to differentiate benign and malignant GGO nodules is inconsistent (21,22). Zhou et al. (23) retrospectively analyzed 58 patients to differentiate minimally invasive adenocarcinoma, and the study demonstrated that pre-invasive or minimally invasive adenocarcinoma manifested as GGO (P<0.01) compared to the invasive adenocarcinoma group. The sensitivity in predicting invasive adenocarcinoma was superior if the consolidation/tumor ratio was greater than 0.38 and the SUVmax greater than 1.46 in mixed GGO nodule (P>0.05) (Figure 3). Recently some authors performed a study in 26 lung cancer patients during free breathing using a 24-min list-mode acquisition on a PET/CT scanner (24). The information were taken using three methods: OG, standard 3D-PET, and respiratory-correlated 4D-PET.
Maximum SUV in the principal tumor, volumes and noise characteristics were compared using threshold of SUV 2.5 and 40% of the SUVmax. The authors concluded that OG PET is an improved alternative to both 3D and 4D PET.
Preoperative radiologic localization and marking
In the real world surgeons know that the localization of a pure GGO during VATS is not easy by finger palpation, and the localization can also be time consuming even at thoracotomy. Localization is more difficult if the nodule is deep in the lung parenchima because the nosiness with vascular and hilar structures, and a 54% failure to palpate the lump by VATS approach. Failure augmented to 63% when the nodule was <10 mm and profound in the pulmonary parenchima. To locate ground-glass opacities can be impossible even by open approach, and therefore a precise localization should be performed preoperatively.
Suspicious nodules may have to be surgically removed via video-assisted thoracoscopic surgery for diagnosis or as a final treatment. Nevertheless, GGO are not easy to locate due to dimension and morphology. Reported evidence regarding markers is consequent from studies on solid nodules <15 mm which are located peripherally. CT-guided injection of 0.2 mL of methylene blue at the periphery of the nodule in combination with a small amount of dye at the subpleural region at the level of the nodule certainly help the surgeon. In a recent prospective randomized trial, CT-guided percutaneous placement of microcoil markers in combination with fuoroscopic-guided VATS resection was significantly better than procedures in which nodule localization was via finnger palpation only in small (mean nodule diameter, 12 mm) solid and subsolid nodules (93% vs. 48%; P<0.01). Other techniques available are intraoperaoperative ultrasonography, hook wire placement, injection of lipidol, and injection of radioisotope, even though this should be the diagnostic approach of last resort.
There is no consensus on which of the several pre-operative localization techniques described is preferred. Moreover the technique could be different according to the surgical approach adopted (25-28).
Indications for surgery for ELC based on imaging pattern only
In the modern era it is very unusual to operate a patient with lung cancer without a proven histology, nevertheless in the real life of a surgeon it can happen for various reasons (location of the tumor, negative histology at biopsy, severe emphysematous lung etc.), and this is happening more frequently with GGO and early lung cancer. Altorki et al. described actuarial survivals between lobectomy (85%) and sublobar resection (86%) in 347 patients with initial stage NSCLC (<3.0 cm) who underwent lobectomy (n=294) or sublobar resection (n=53) (P=0.86) (29), and no difference was found.
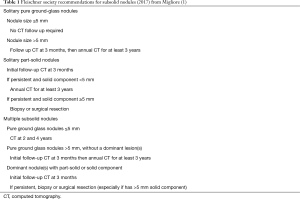
Many guidelines have been recently published: Fleischner Society (Table 1, modified from Migliore et al.) (1), British thoracic society and the Japan Clinical Oncology Group (JCOG) (30-32).
The proposal of the JCOG for GGO reports that lobectomy is indicated for GGO >3.0 cm, segmentectomy for nodules >2 and <3 cm, and wedge resection for GGO <2 cm. Mixed and bigger GGOs with substantial solid component, and associated higher CEA are more often associated with nodal metastasis, and more extended surgery should be performed.
Discussion
We should emphasise that this is a time that reflect the spirit of innovation of our specialty as seldom before, and a preoperative diagnosis of indeterminate pulmonary lesions, partially solid nodules and pure ground glass is mandatory in view of the increasing number of segmental resections for early stage lung cancer.
It is evident that excisional biopsy by mean of video-assisted thoracic surgery (VATS) is a viable choice but the localization of these lesion intra-operatively is often impossible without localization techniques and when using a completely portal approach (25-28). Localization can be performed in a different ways using devices such as hookwires, microcoils, dye, collagen, radioactive labeled solutions.
Hookwires, known to mark breast lesions, has been adopted by interventional radiologists and thoracic surgeons to localize lung tumor. An Italian study on 151 patients with pulmonary nodules <1 cm and deep in the lung parenchyma >1.5 cm underwent CT- guided wire localization prior to VATS removal, and 7.5% had a pneumothorax while 7.5% dislodged the hookwire prior to resection (33). As localization can be performed in <45 min in 95% of lesions, the need for conversion to thoracotomy is reduced (4.7%). Pneumothorax is reported in 38% of procedures but only occasionally a chest drain is required. Moreover navigational bronchoscopy (34,35) is a promising tool in order to localize the lesion using ethylene and patent blue, indocyanine green, collagen and lipiodol. The use of 99mTechnetium injection for radioactive labeling adjacent to pulmonary nodules have been successfully trialed.
Conclusions and future perspectives
Screening program with low-dose CT led to an increased detection of early stage lung cancer and GGO. Only an accurate preoperative radiologic imaging permits to localize the lesion and individualize the proper surgical treatment of ELC such as segmentectomy or lobectomy, in the future hybrid operating rooms will become an essential tool in thoracic surgery.
Furthermore, the development of 3D imaging brought to the precise preoperative evaluation of anatomical structures for lung cancer surgery such as bronchi and vessels which in turn has permitted surgeons to carry out safer and more precise VATS. Moreover, 3D imaging technology has the potential to predict the molecular features of lung cancer without the need for pathological examination. Finally, for the above mentioned reasons the MDT meeting between surgeons and radiologists in the decision making of early lung cancer and GGO is mandatory and should follow the EBM rules, as the professional opinion in these circumstances is not enough (36).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mario Nosotti, Ilaria Righi and Lorenzo Rosso) for the series “Early Stage Lung Cancer: Sublobar Resections are a Choice?” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.02.61). The series “Early Stage Lung Cancer: Sublobar Resections are a Choice?” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Migliore M, Fornito M, Palazzolo M, et al. Ground glass opacities management in the lung cancer screening era. Ann Transl Med 2018;6:90. [Crossref] [PubMed]
- Sone S, Li F, Yang ZG, et al. Characteristics of small lung cancers invisible on conventional chest radiography and detected by population based screening using spiral CT. Br J Radiol 2000;73:137-45. [Crossref] [PubMed]
- Koo HJ, Choi CM, Park S, et al. Chest radiography surveillance for lung cancer: Results from a National Health Insurance database in South Korea. Lung Cancer 2019;128:120-6. [Crossref] [PubMed]
- Kazerooni EA, Austin JH, Black WC, et al. ACR-STR practice parameter for the performance and reporting of lung cancer screening thoracic computed tomography (CT): 2014 (Resolution 4). J Thorac Imaging 2014;29:310-6. [Crossref] [PubMed]
- Silva M, Pastorino U, Sverzellati N. Lung cancer screening with low-dose CT in Europe: strength and weakness of diverse independent screening trials. Clin Radiol 2017;72:389-400. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low- dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. [Crossref] [PubMed]
- Rampinelli C, Origgi D, Bellomi M. Low-dose CT: technique, reading methods and image interpretation. Cancer Imaging 2013;12:548-56. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Li F, Sone S, Abe H, et al. Lung cancers missed at low-dose helical CT screening in a general population: comparison of clinical, histopathologic, and imaging findings. Radiology 2002;225:673-83. [Crossref] [PubMed]
- Henschke CI, Yankelevitz DF, Mirtcheva R, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 2002;178:1053-7. [Crossref] [PubMed]
- Caminati A, Cavazza A, Sverzellati N, et al. An integrated approach in the diagnosis of smoking-related interstitial lung diseases. Eur Respir Rev 2012;21:207-17. [Crossref] [PubMed]
- Kim HY, Shim YM, Lee KS, et al. Persistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisons. Radiology 2007;245:267-75. Erratum in: Radiology 2008;247:297. [Crossref] [PubMed]
- Hasegawa M, Sone S, Takashima S, et al. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol 2000;73:1252-9. [Crossref] [PubMed]
- Lee HY, Lee KS. Ground-glass opacity nodules: histopathology, imaging evaluation, and clinical implications. J Thorac Imaging 2011;26:106-18. [Crossref] [PubMed]
- Sardari Nia P, Olsthoorn JR, Heuts S, et al. Interactive 3D Reconstruction of Pulmonary Anatomy for Preoperative Planning, Virtual Simulation, and Intraoperative Guiding in Video-Assisted Thoracoscopic Lung Surgery. Innovations 2019;14:17-26. [PubMed]
- Migliore M, Calvo D, Criscione A, et al. Lung cancer invading a single left pulmonary vein requiring extended pneumonectomy. Future Oncol 2016;12:55-7. [Crossref] [PubMed]
- Kudo Y, Ikeda N. Benefits of lung modeling by high-quality three-dimensional computed tomography for thoracoscopic surgery. Video-assist Thorac Surg 2019;4:4. [Crossref]
- Chan EG, Landreneau JR, Schuchert MJ, et al. Preoperative (3-dimensional) computed tomography lung reconstruction before anatomic segmentectomy or lobectomy for stage I non–small cell lung cancer. J Thorac Cardiovasc Surg 2015;150:523-8. [Crossref] [PubMed]
- Ebara K, Takashima S, Jiang B, et al. Pleural invasion by peripheral lung cancer: prediction with three-dimensional CT. Acad Radiol 2015;22:310-9. [Crossref] [PubMed]
- Akiba T, Inagaki T, Nakada T. Three-dimensional printing model of anomalous bronchi before surgery. Ann Thorac Cardiovasc Surg 2014;20:659-62. [Crossref] [PubMed]
- Furumoto H, Shimada Y, Imai K, et al. Prognostic impact of the integration of volumetric quantification of the solid part of the tumor on 3DCT and FDG-PET imaging in clinical stage IA adenocarcinoma of the lung. Lung Cancer 2018;121:91-6. [Crossref] [PubMed]
- Eriguchi D, Shimada Y, Imai K, et al. Predictive accuracy of lepidic growth subtypes in early-stage adenocarcinoma of the lung by quantitative CT histogram and FDG-PET. Lung Cancer 2018;125:14-21. [Crossref] [PubMed]
- Zhou J, Li Y, Zhang Y, et al. Solitary ground-glass opacity nodules of stage IA pulmonary adenocarcinoma: combination of 18F-FDG PET/CT and high-resolution computed tomography features to predict invasive adenocarcinoma. Oncotarget 2017;8:23312-21. [Crossref] [PubMed]
- van Elmpt W, Hamill J, Jones J, et al. Optimal gating compared to 3D and 4D PET reconstruction for characterization of lung tumours. Eur J Nucl Med Mol Imaging 2011;38:843-55. [Crossref] [PubMed]
- Chella A, Lucchi M, Ambrogi MC, et al. A pilot study of the role of TC-99 radionuclide in localization of pulmonary nodular lesions for thoracoscopic resection. Eur J Cardiothorac Surg 2000;18:17-21. [Crossref] [PubMed]
- Nardini M, Bilancia R, Paul I, et al. 99mTechnetium and methylene blue guided pulmonary nodules resections: preliminary British experience. J Thorac Dis 2018;10:1015-21. [Crossref] [PubMed]
- Migliore M, Deodato G. A single-trocar technique for minimally invasive surgery of the chest. Surg Endosc 2001;15:899-901. [Crossref] [PubMed]
- Migliore M. Efficacy and safety of single-trocar technique for minimally invasive surgery of the chest in the treatment of noncomplex pleural disease. J Thorac Cardiovasc Surg 2003;126:1618-23. [Crossref] [PubMed]
- Altorki NK, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg 2014;147:754-62. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017. [Crossref] [PubMed]
- Baldwin DR, Callister MEJ. The British Thoracic Society guidelines on the investigation and management of pulmonary nodules. Thorax 2015;70:794-8. [Crossref] [PubMed]
- Aokage K, Saji H, Suzuki K, et al. Lung Cancer Surgical Study Group of the Japan Clinical Oncology Group. A non-randomized confirmatory trial of segmentectomy for clinical T1N0 lung cancer with dominant ground glass opacity based on thin-section computed tomography (JCOG1211). Gen Thorac Cardiovasc Surg 2017;65:267-72. [Crossref] [PubMed]
- Ciriaco P, Negri G, Puglisi A, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429-33. [Crossref] [PubMed]
- Anantham D, Feller-Kopman D, Shanmugham LN, et al. Electromagnetic navigation bronchoscopy-guided fiducial placement for robotic stereotactic radiosurgery of lung tumors: a feasibility study. Chest 2007;132:930-5. [Crossref] [PubMed]
- Eberhardt R, Anantham D, Herth F, et al. Electromagnetic navigation diagnostic bronchoscopy in peripheral lung lesions. Chest 2007;131:1800-5. [Crossref] [PubMed]
- Treasure T, Utley M, Hunt I. When professional opinion is not enough. Brit Med J 2007;334:831-2. [Crossref] [PubMed]