
Clinical characteristics and treatment outcomes of patients with pneumonia caused by Raoultella planticola
Introduction
Raoultella planticola has been considered to be a relatively harmless environmental Gram-negative bacterium rarely associated with human clinical infections. However, in recent years, the frequency of severe R. planticola infection reported in the literature has increased (1). This environmental organism, which infrequently causes human infections, is pathogenically similar to Klebsiella spp., which is associated with severe infections (bacteremia, pneumonia, and urinary tract infections) in hospitalized and immunocompromised patients; Klebsiella is responsible for 3–7% of all nosocomial infections (2). Although reports on conjunctivitis, liver abscesses, cholangitis, pancreatitis, prostate conditions, necrotizing fasciitis, and bacteremia caused by R. planticola have appeared (3-9), R. planticola pneumonia remains rare. To the best of our knowledge, only five cases have been reported in the English-language literature (10-14). The clinical characteristics and outcomes of such infections remain to be investigated. Here, we evaluated the clinical characteristics, management, and clinical outcomes of patients with pneumonia caused by R. planticola.
Methods
This was a retrospective chart review of patients with pneumonia caused by R. planticola evaluated and treated at Dankook university hospital (a 809-bed referral hospital in Cheoan, South Korea) between January 2011 and December 2017. All pulmonary secretion isolates reported as R. planticola were selected and the medical records of these patients were retrieved. The clinical features of the infections were reviewed, and cases with symptoms indicative of pneumonia were included in the present study. We recorded gender, age, any underlying disease, radiographic features, the number of sputum cultures yielding R. planticola, the antimicrobial regimen administered, any microbes other than R. planticola that were isolated, and outcomes. Clinical infections were defined using established criteria (15). Community-acquired pneumonia (CAP), ventilator-associated pneumonia (VAP), bloodstream infections (BSIs), sepsis, severe sepsis, and septic shock were defined by reference to the Center for Disease Control and American Thoracic Society (ATS) clinical diagnostic criteria (16-19). Pneumonia was defined as the presence of “[a] new lung infiltrate plus clinical evidence that the infiltrate is of an infectious origin, which include[s] the new onset of fever, purulent sputum, leukocytosis, and decline in oxygenation” (16-18,20). Chest radiography and computed tomography (CT) scans were reviewed by an experienced radiologist and a single pulmonologist, and consensus diagnoses were attained. We evaluated consolidation, ground-grass opacity (GGO), micronodule status, and pleural effusion.
All kinds of specimens including blood, pulmonary secretion (sputum, tracheal aspirate), urine, pleural fluid (if patients had pleural effusion) were examined for microbiological examination such as urinary antigen test for Legionella pneumophila or Streptococcus pneumoniae, serologic test for Mycoplasma, Chlamydia, Coxiella, polymerase chain reaction (PCR) for tuberculosis, respiratory viruses, culture for acid-fast bacilli (AFB), bacteria and fungus from patients with suspected pneumonia. Especially, pulmonary secretions underwent microscopic and bacteriological examinations; we engaged in both non-invasive sampling (sputum expectoration and endotracheal aspiration) and invasive sampling (bronchoscopic bronchial washing). An adequate (“good-quality”) sputum sample exhibited a polymorphonuclear cell:epithelial cell ratio of 25:10 at a magnification of 100× (21). Endotracheal aspirates samples were subjected to quantitative culture using ≥106 colony-forming units (CFU)/mL as cutoffs (18). Microbe identification and antibiotic susceptibility tests were performed using the automated VITEK® 2 system (bioMe´rieux, Marcyl’E´toile, France) accompanied by routine bacteriologic methods. All isolates were identified with a probability score exceeding 96%. Antimicrobial susceptibility testing was performed as described by the Clinical and Laboratory Standards Institute (22). We recorded clinical characteristics, radiological features, treatments, and clinical outcomes. The need for informed consent was waived as the work was retrospective in nature. The study was approved by the institutional review board of our hospital.
Results
Clinical characteristics
Overall, 63 patients yielded positive culture results for R. planticola in blood, urine, bile, peritoneal fluid, pus, pleural fluid, and/or sputum samples (including those obtained via tracheal aspiration) over the 7-year period. Of these, 24 yielded sputum/tracheal aspirate-positive cultures and 11 met the diagnostic criteria for pneumonia. Most isolates were from sputum (7/11, 63.6%); four were from tracheal aspirates. In seven cases, R. planticola was the sole microbial isolate; microbiological examination was noted no bacteria except for R. planticola, no viruses, no fungus, and negative AFB smear; in four, additional microbes were also isolated only in the pulmonary secretion, reflecting either airway colonization or contamination. The number of pneumonia cases was 1–2 annually (average =1). Of the 11 patients, 10 were females and the median age was 70 years (range: 51–79 years). The major underlying disease was malignancy (five patients). Two patients had solid tumors in, or metastases to, the pancreas, gallbladder, or bile duct; two had lung cancer; and one had soft palate cancer. Three patients had been treated for cerebral infarction or hemorrhage and two had chronic obstructive pulmonary disease. The patients are described in chronological order in Table 1.
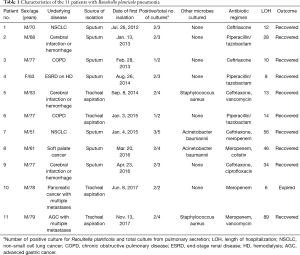
Full table
Radiographic features
Chest radiographs were available for all patients and chest CT scans for 10 (90.9%). Consolidation (8/11, 72.7%), GGO (5/11, 45.5%), pleural effusion (5/11, 45.5%), and micronodules (3/11, 27.3%) were the most common findings. Diffuse GGO was predominant in three patients. Within 1 week after illness onset, focal consolidation was the predominant finding in six patients, whereas two evidenced multiple bilateral consolidations accompanied by GGO. The right and left lower lobes were most commonly involved (n=7 for both), followed by the right middle lobe (n=3), left upper lobe (n=2), and right upper lobe (n=2). The typical radiologic findings of the patients are described in the Table 2.
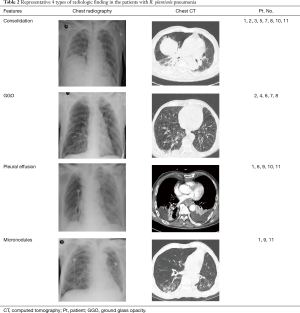
Full table
Treatments and clinical outcomes
All patients were admitted; the median hospitalization time was 14 days (range: 6–89 days). Four (36.4%) patients (patients 5, 6, 10, and 11) required mechanical ventilation of median duration 5 days (range: 4–12 days); of these patients, three survived but one (patient 10) died of multiple organ dysfunction syndrome (principally pneumonia and septic shock). Hospital-acquired pulmonary infections (principally Acinetobacter baumannii) developed in three patients (patients 7, 8, and 11). No patient received systemic steroids. In seven cases, R. planticola was the sole microbial isolate; in four cases, although additional microbes (Staphylococcus aureus or Acinetobacter baumannii) were isolated in their sputum or tracheal aspiration specimens, R. planticola were isolated at the same time when they had symptoms or signs of pneumonia, whereas Staphylococcus aureus or Acinetobacter baumannii were isolated later when antibiotics treatment for R. planticola was in progress. Antimicrobial susceptibility testing revealed that all isolates were resistant to ampicillin, but became susceptible when a β-lactamase inhibitor was added. All isolates were susceptible to cephalosporins, all third-generation cephalosporins, carbapenems, fluoroquinolones, tigecycline, and trimethoprim/sulfamethoxazole.
Empirical, broad-spectrum intravenous antibiotics were given to all patients. The antimicrobial agent most frequently used was ceftriaxone (45.5%, 5/11), followed by piperacillin/tazobactam (27.3%, 3/11) and carbapenems (27.3%, 3/11). When positive culture results for R. planticola and polymicrobial hospital-acquired pathogens were available, the antimicrobial treatment agents were modified in 36.4% (4/11) of patients. Culture study for pulmonary secretion such as sputum and/or tracheal aspirates samples performed every five to seven days and there was no change in the antimicrobial susceptibility after some days of antibiotic treatment in the all patients. The targeted antimicrobial agents most frequently used were ceftriaxone and piperacillin/tazobactam. Most patients (10/11, 91%) survived infection.
Discussion
R. planticola is an aerobic, non-capsulated, non-motile Gram-negative bacillus of the Enterobacteriaceae initially classified as Klebsiella planticola or K. trevisanii. Further phylogenetic analysis revealed major differences among the eight species of Klebsiella (23). In 2001, a new taxonomy was established based on comparative analyses of the 16S rRNA and rpoB genes, resulting in the creation of a new genus, Raoultella, which includes R. planticola, R. ornithinolytica, and R terrigena (24,25). R. planticola does not typically infect humans. Several studies estimate that between 9–18% of humans are colonized with the bacterium (25,26). As infection is rare, risk factors associated with infection are largely inferred from case reports. These include an immunocompromised state, invasive medical procedures, seafood consumption, and exposure to aquatic or soil contaminants. Chun et al. retrospectively studied 20 Korean patients with R. planticola bacteremia and found that 14 from whom R. planticola was the sole microbial isolate recovered after prescription of empirical antibiotics. Of six patients with polymicrobial infections, three died. Antimicrobial susceptibility testing revealed that all strains were susceptible to β-lactamase inhibitors, all tested third-generation cephalosporins, carbapenems, and fluoroquinolone agents (27). Although infections caused by R. planticola have increased in number, R. planticola pneumonia remains rare. The first case, described by Castanheira et al., involved an 83-year-old female hospitalized to treat CAP. She eventually died of pneumonia and septic shock (10). Tseng et al. and Xu et al. reported pneumonia caused by a carbapenem-resistant R. planticola strain isolated from sputum culture (11,12). More recently, two case reports described patients with pneumonia caused by primary infection with R. planticola susceptible to most antibiotics, including cephalosporins, fluoroquinolones, aminoglycosides, and carbapenems (13,14). We found that all R. planticola isolates were susceptible to most antibiotics; most patients experienced good outcomes and only one patient, who required mechanical ventilation, died.
In general, R. planticola is sensitive to a wide range of antibiotics. However, like Klebsiella spp., R. planticola can acquire plasmid-borne antibiotic-resistance genes triggering severe, and even fatal, infections (10-12). Carbapenem resistance in R. planticola involves the production of carbapenemases, including class A β-lactamase (KPC), class B metal-β-lactamase (IMP-8, NDM-1), and class D β-lactamase (OXA-48) (10,11). Notably, bla KPC, bla IMP-8, and bla NDM-1 are usually located on plasmids or transposons, suggesting possible genetic exchange between R. planticola and other Enterobacteriaceae, such as K. pneumoniae (12). There were very few cases of pneumonia caused by R. planticola which could confirm imaging findings. In that cases, it had appeared in various radiologic finding such as consolidation (14), consolidation with pleural effusion (12), and diffuse GGO with consolidation (13). The present study showed that consolidation, GGO, pleural effusion, and micronodules were the most common findings in that order.
R. planticola may infect patients after a traumatic incident in a contaminated environment. Nosocomial infection involves bacterial introduction during an invasive hospital procedure; either dormant colonizers are activated or the instruments are contaminated. Systemic impairment of the host immune system enables dormant colonizers to become invasive. However, enteric fever and bacteremia have been recorded in an immunocompetent patient (1). We found it difficult to distinguish between pathogenicity and colonization in some cases; however, pulmonary secretions from seven cases grew only R. planticola. Four patients exhibited polymicrobial infections, but it is likely that R. planticola caused the pneumonia because R. planticola emergence coincided with aggravation of the pulmonary infection. Furthermore, the specimens were only adequate; Gram-negative bacilli predominated and bacterial numbers were moderate-to-high in the gram stains and it was also consisted with the result of culture. Hence, it is likely that R. planticola caused the pneumonia.
Our study had the potential limitations associated with a retrospective review design. First, there may have been reviewer bias; however, most of the data were collected by the primary author using a standardized method and diagnostic criteria. Second, gram stains and culture for pulmonary secretion including sputum and tracheal aspiration are most often the first diagnostic method for pneumonia. Therefore, it is very important to achieve the adequacy of specimen. Although this study had been conducted with the adequate specimen, the possibility of colonization cannot be completely excluded. Finally, we evaluated only a small number of patients treated in a single center. However, the clinical course was consistently good even among the patients with complications; early administration of intravenous antibiotics obviates pulmonary catastrophe and ensures survival.
In conclusion, R. planticola may be an emerging pneumonia pathogen that could infect even immunocompetent humans. The bacterium may become multidrug-resistant, exerting an increasingly heavy toll in terms of morbidity and mortality. Pulmonologists should be familiar with the risk factors for R. planticola pneumonia, note the existence of carbapenemase-resistant strains, and promptly diagnose and treat these potentially deadly infections. Further studies are required on this topic.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.02.56). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The need for informed consent was waived as the work was retrospective in nature. The study was approved by the institutional review board of our hospital.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ershadi A, Weiss E, Verduzco E, et al. Emerging pathogen: a case and review of Raoultella planticola. Infection 2014;42:1043-6. [Crossref] [PubMed]
- Olson DS Jr, Asare K, Lyons M, et al. A novel case of Raoultella planticola urinary tract infection. Infection 2013;41:259-61. [Crossref] [PubMed]
- O' Connell K, Kelly J, Niriain U. A rare case of soft-tissue infection caused by Raoultella planticola. Case Rep Med 2010;2010:134086. [Crossref] [PubMed]
- Teo I, Wild J, Ray S, et al. A rare case of cholecystitis caused by Raoultella planticola. Case Rep Med 2012;2012:601641. [Crossref] [PubMed]
- Koukoulaki M, Bakalis A, Kalatzis V, et al. Acute prostatitis caused by Raoultella planticola in a renal transplant recipient: a novel case. Transpl Infect Dis 2014;16:461-4. [Crossref] [PubMed]
- Sitaula S, Shahrrava A, Al Zoubi M, et al. The first case report of Raoultella planticola liver abscess. IDCases 2016;5:69-71. [Crossref] [PubMed]
- Vassallo J, Vella M, Cassar R, et al. Four cases of Raoultella planticola conjunctivitis. Eye (Lond) 2016;30:632-4. [Crossref] [PubMed]
- Alves MS, Riley LW, Moreira BM. A case of severe pancreatitis complicated by Raoultella planticola infection. J Med Microbiol 2007;56:696-8. [Crossref] [PubMed]
- Hu AY, Leslie KA, Baskette J, et al. Raoultella planticola bacteraemia. J Med Microbiol 2012;61:1488-9. [Crossref] [PubMed]
- Castanheira M, Deshpande LM, DiPersio JR, et al. First descriptions of blaKPC in Raoultella spp. (R. planticola and R. ornithinolytica): report from the SENTRY antimicrobial surveillance program. J Clin Microbiol 2009;47:4129-30. [Crossref] [PubMed]
- Tseng SP, Wang JT, Liang CY, et al. First report of bla(IMP-8) in Raoultella planticola. Antimicrob Agents Chemother 2014;58:593-5. [Crossref] [PubMed]
- Xu M, Xie W, Fu Y, et al. Nosocomial pneumonia caused by carbapenem-resistant Raoultella planticola: a case report and literature review. Infection 2015;43:245-8. [Crossref] [PubMed]
- Cho YJ, Jung EJ, Seong JS, et al. A case of pneumonia caused by Raoultella planticola. Tuberc Respir Dis (Seoul) 2016;79:42-5. [Crossref] [PubMed]
- Westerveld D, Hussain J, Aljaafareh A, et al. A rare case of Raoultella planticola pneumonia: An emerging pathogen. Respir Med Case Rep 2017;21:69-70. [Crossref] [PubMed]
- Garner JS, Jarvis WR, Emori TG, et al. CDC definitions for nosocomial infections, 1988. Am J Infect Control 1988;16:128-40. [Crossref] [PubMed]
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27-72. [Crossref] [PubMed]
- Kollef MH. Diagnosis of ventilator-associated pneumonia. N Engl J Med 2006;355:2691-3. [Crossref] [PubMed]
- American Thoracic Society. Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare associated pneumonia. Am J Respir Crit Care Med 2005;171:388-416. [Crossref] [PubMed]
- Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med 2003;31:1250-6. [Crossref] [PubMed]
- Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61-111. [Crossref] [PubMed]
- Murray PR, Washington JA II. Microscopic and bacteriologic analysis of expectorated sputum. Mayo Clin Proc 1975;50:339-44. [PubMed]
- CLSI. M100-S21. Performance standards for antimicrobial susceptibility testing. 2012;21st informational supplement.
- Bagley S, Seidler R, Brenner D. Klebsiella planticola sp. nov.: a new species of enterobacteriaceae found primarily in nonclinical environments. Curr Microbiol 1981;6:105-9. [Crossref]
- Drancourt M, Bollet C, Carta A, et al. Phylogenetic analyses of Klebsiella species delineate Klebsiella and Raoultella gen. nov., with description of Raoultella ornithinolytica comb.nov., Raoultella terrigena comb. nov. and Raoultella planticola comb. nov. Int J Syst Evol Microbiol 2001;51:925-32. [Crossref] [PubMed]
- Podschun R, Acktun H, Okpara J, et al. Isolation of Klebsiella planticola from newborns in a neonatal ward. J Clin Microbiol 1998;36:2331-2. [Crossref] [PubMed]
- Westbrook GL, O’Hara CM, Roman SB, et al. Incidence and identification of Klebsiella planticola in clinical isolates with emphasis on newborns. J Clin Microbiol 2000;38:1495-7. [Crossref] [PubMed]
- Chun S, Yun JW, Huh HJ, et al. Low virulence? Clinical characteristics of Raoultella planticola bacteremia. Infection 2014;42:899-904. [Crossref] [PubMed]


