Adjuvant therapy in stage IIIA-N2 non-small cell lung cancer after neoadjuvant concurrent chemoradiotherapy followed by surgery
Introduction
Approximately 20% of all non-small cell lung cancer (NSCLC) is stage IIIA-N2 (1). Those patients are heterogeneous in terms of disease extent and survival. Treatment options included induction chemotherapy or concurrent chemoradiotherapy (CCRT) followed by surgery, or definitive CCRT (2-7). The optimal strategy for IIIA-N2 NSCLC remains controversial and no treatment is clearly recommended (8,9).
The overall prognosis of IIIA NSCLC is still poor, with a median survival time of 14–25 months despite multimodal therapy. Many patients develop local or distant recurrence eventually many patients develop local and distant recurrence (8-11). Additional adjuvant therapy may be needed to control the disease, although the benefit of adjuvant treatment after multimodal therapy is doubtful owing to the considerable toxicity of each therapy (12).
There are limited data on adjuvant therapy for patients who have undergone neoadjuvant CCRT and surgical treatment. Therefore, we examined the effects of the adjuvant therapy in patients with stage IIIA-N2 NSCLC after neoadjuvant CCRT followed by surgery.
Methods
Study population
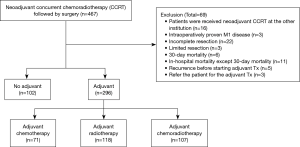
We retrospectively reviewed the medical records of all patients who underwent surgery for NSCLC with curative intent surgery at the Samsung Medical Center a 1,961-bed referral hospital in Seoul, South Korea, between January 2004 and December 2013. During this period, 467 patients underwent neoadjuvant CCRT followed by surgical resection for stage IIIA-N2 NSCLC. Of these, patients who received CCRT at our institution and followed by complete resection with systematic lymph node (LN) dissection were included in this study population. Exclusion criteria were summarized in Figure 1. Ultimately, 398 patients were included: 102 patients did not receive the adjuvant therapy after surgery (no-therapy group) and 296 patients received adjuvant treatment including chemotherapy (n=71), radiotherapy (n=118) or chemoradiotherapy (CRT) (n=107) (therapy group). To review and publish the information obtained from patient records, the study was given approval by the Institutional Review Board of Samsung Medical Center (IRB No. 2015-05-143).
Preoperative staging work-up
The routine preoperative workup included pulmonary function tests, computed tomography (CT) scans of the chest and upper abdomen, positron emission tomography (PET)/CT scans, flexible bronchoscopy, and magnetic resonance imaging (MRI) of the brain. Preoperatively, 360 patients were pathologically confirmed to have mediastinal LN metastasis. Mediastinoscopy (n=213) and endobronchial ultrasound transbronchial needle aspiration (EBUS-TBNA) (n=124), were performed most frequently to prove LN metastasis, although video-assisted thoracic surgery (VATS) LN biopsy (n=13), combined VATS and mediastinoscopy (n=4), anterior mediastinotomy (n=3), combined mediastinoscopy and mediastinotomy (n=3) were also performed. For the remaining patients, mediastinal staging was based on CT and PET/CT findings without pathologic confirmation.
Neoadjuvant CCRT
Neoadjuvant CCRT included chemotherapy and concurrent thoracic radiotherapy. The therapeutic regimens of were different according to year of administration. Before October 2009, radiation therapy was delivered to patients with a total dose of 45 Gy (1.8 Gy/fraction/day) over 5 weeks. From October 2009 and thereafter, radiation dose was 44Gy (2.0 Gy/fraction/day) over 4.5 weeks using 10-MV X-rays. The chemotherapy regimen mostly consisted of weekly paclitaxel (50 mg/m2 per week, iv) or docetaxel (20 mg/m2 per week, iv) plus cisplatin (25 mg/m2 per week, iv) or carboplatin (AUC 1.5/week, iv) for 5 weeks.
Surgery
Surgical resection was scheduled at 4–6 weeks following the completion of neoadjuvant CCRT. Operative procedures included lobectomies, bilobectomies, or pneumonectomies as indicated. Mediastinal LN dissection consisted of en bloc resections of all nodes at stations 2R, 4R, 7, 8, and 9 and 10R for a right-sided tumor and 4L, 5, 6, 7, 8, and 9 and 10L for a left-sided tumor.
Postoperative treatment and follow-up
Postoperative treatment was decided using multidisciplinary team approach after considering the extent of disease and general condition of each patient. Postoperative treatment included chemotherapy (taxane or vinorelbine, combined with platinum), radiotherapy (18 Gy in 10 fraction), CRT or no treatment. Patients were regularly evaluated by chest CT scans every 3 to 4 months for the first 2 years following surgery and every 6 months thereafter. Patients were annually evaluated by PET/CT scans for detection of recurrence.
Statistical analysis
The baseline characteristics assessing were compared using student t-test or ANOVA for continuous variables and chi-square or Fisher’s exact test for categorical variables. Overall survival (OS) was defined as the time from the date of surgery until the last date of follow-up for patients who remained alive or until death. Disease-free survival (DFS) was defined as the time from the date of surgery to recurrence or death. The Kaplan-Meier method with the log-rank test and Cox proportional hazards model were conducted to determine the prognostic impact of adjuvant therapy. All statistical tests were two-sided with a significance level set at 0.05 and were performed using PASW Statistic 21 (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics
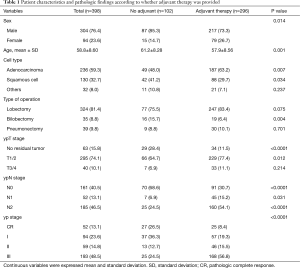
Table 1 summarized the baseline characteristics according to whether adjuvant therapy was provided. The mean age was 59 years (range, 32–76 years) and the majority of patients were males. Patients in the adjuvant therapy group were significantly younger (61.2 vs. 57.9, P=0.001) and had a greater percentage of female (P=0.014) compared to those in the no adjuvant therapy group. Adenocarcinoma was the predominant cell type regardless of postoperative treatment; the incidence of adenocarcinoma and squamous cell carcinoma was significantly different between the two groups. In terms of type of surgery, lobectomy was the most common. More lobectomies tended to be performed in the adjuvant therapy group (P=0.075), while significantly more bilobectomies were performed in the no adjuvant therapy group (P=0.004).
Full table
Pathologic response according to adjuvant therapy
The postoperative pathologic findings are showed in Table 1. Mediastinal clearance was achieved in 213 patients (53.6%), whereas residual N2 disease was present in 185 patients (46.5%). More than half of patients (n=161) in the adjuvant therapy group had residual mediastinal metastasis, while only 25 patients had ypN2 disease in the no adjuvant therapy group. There was a significant difference in the mediastinal clearance between the two groups (P<0.0001). A complete pathologic response was observed in 52 patients (13.1%). Patients in the no adjuvant therapy group had significantly more pathologic response compare to the adjuvant therapy group (P<0.0001).
Patient characteristic and pathologic findings across adjuvant treatment types
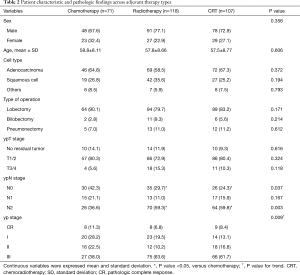
As shown in Table 2, age, sex and histologic type were similar across the type of adjuvant therapy, whereas ypN stage (for N0, P=0.037; for N2, P=0.003) and overall yp stage (P=0.009) were significantly higher in the radiotherapy or CRT groups compare to the chemotherapy group.
Full table
OS/DFS with adjuvant therapy
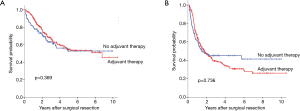
Kaplan-Meier curves and the log-rank test showed no significant differences in OS (Figure 2A, P=0.369) and DFS (Figure 2B, P=0.736) between patients with or without adjuvant therapy. The 5-year OS was 54.9% in the adjuvant therapy group and 52.9% in the no adjuvant therapy group; the values for 5-year DFS were 30.7% and 45.1%, respectively.
In regards to yp stage, 5-year OS was significantly different by yp stage (pathologic complete response,72.3%; stage I,66.2%; stage II, 54.8% and stage III, 41.4%; P=0.029) (Figure 3A). Adjuvant therapy was associated with significantly better OS in patient with yp stage II (Figure 3B,C,D,E, 19.7% vs. 61.8%, P=0.008). Also, DFS differed according to yp stage (CR, 64.6%; stage I, 50.8%; stage II, 28.5% and stage III, 17.8%, P<0.0001) (Figure 4A). Patients with yp stage III is likely to have survival benefit with adjuvant treatment (DFS, 16.7% vs. 18.7%, P=0.036), otherwise statistical significance was not found (Figure 4B,C,D,E).
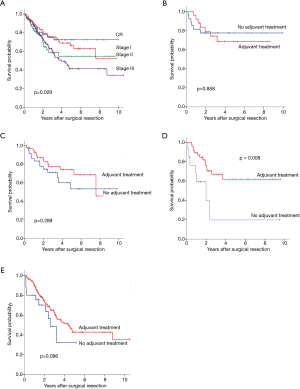
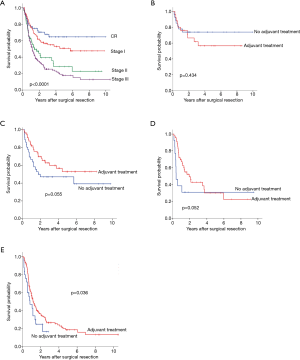
Prognostic impact of adjuvant therapy/type of adjuvant therapy for OS and DFS
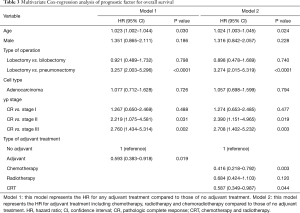
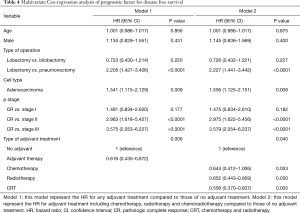
After adjusting for age, sex, type of operation, cell type and yp stage, adjuvant therapy was associated with a significantly better OS [hazard ratio (HR) =0.593; 95% CI, 0.383–0.918; P=0.019] (Table 3) and DFS (HR =0.616; 95% CI, 0.435–0.872; P=0.006) compared to patients in the no adjuvant therapy group (Table 4).
Full table
Full table
In terms of type of adjuvant treatment, adjuvant chemotherapy and CRT were associated with a significantly better OS (adjuvant chemotherapy; HR =0.416; 95% CI, 0.22–0.79; P=0.003, adjuvant CRT; HR =0.587; 95% CI, 0.349–0.987; P=0.044) in the multivariable analysis (Table 3), while adjuvant radiotherapy and CRT were associated with a better DFS after adjusting for clinical variables (Table 4).
Discussion
The OS rate and DFS rate were similar whether adjuvant therapy was given to patients who received neoadjuvant CCRT with subsequent complete surgical resection for stage IIIA-N2 NSCLC, although patients in the no adjuvant therapy group had better pathologic response to neoadjuvant treatment compare to patients in the adjuvant therapy group. Remarkably, a Cox-regression model showed that adjuvant therapy was independent prognostic factors related to better OS and DFS even after adjustment of sex, age, histology, surgical procedure and pathologic stage.
Several factors have been described to predict survival after neoadjuvant treatment followed by surgery. Mediastinal downstaging and regression of the primary tumor is associated with improved survival compared with patients with residual N2 disease (8,9,13-18). Previously, we obtained a similar result; the ypN stage was significant prognostic factor of OS ad DFS (19). Single node station, minimal N2 disease and decrease in intensity of uptake on PET scan are also considered favorable prognostic factors (8,9,20,21).
Cisplatin-based adjuvant chemotherapy has become the standard for patients with completely resected stage II or III NSCLC (22-24). Postoperative radiotherapy is controversial and not recommended routinely for patients with completely resected N2 NSCLC. A study based on the Surveillance, Epidemiology, and End Results (SEER) database and Adjuvant Navelbine International Trialist Association (ANITA) trial demonstrated the benefit of postoperative radiotherapy for N2 disease after surgical resection (25,26). However, for patients who underwent neoadjuvant CCRT, the role of adjuvant chemotherapy or radiotherapy was not known.
To the best of our knowledge, this is the first investigation to document the impact of adjuvant therapy for patients who underwent trimodal therapy for stage IIIA-N2 NSCLC. The overall 5-year OS was 54%, and according to ypN stage, the OS in patients with N0, N1 and N2 disease was 65.8%, 58.5% and 40.8%, respectively. These outcomes are quite favorable compared to the reported survival rate of patients with ypN2 disease of 9–29% (4,6,9,27-29).
At our institution, selected patients were given adjuvant treatment after trimodal therapy. The extent of disease was the critical factor when deciding whether to give adjuvant therapy. Using a multidisciplinary team approach, each patient was evaluated to determine the risks and benefits of adjuvant treatment. If the patient was deemed able to tolerate further treatment, we recommended adjuvant therapy, especially for patients with persistent mediastinal disease, which is a known prognostic factor. Typically, patients who was expected favorable outcomes (less extent of disease) and relatively healthy patients might undergo surgical resection. Therefore, our survival results are not applicable to all patients with N2 disease. In the subgroup analysis according to yp stage, adjuvant therapy resulted in significantly better survival in patients with yp stage II and better DFS in patients with yp stage III. This suggests that the impact of adjuvant treatment differs according to yp or ypN stage. Overall, our findings suggest that adjuvant therapy is beneficial in selected patients who have a poor pathologic response. They might also explain the good survival outcomes in patients with ypN1 or N2 disease.
There are several limitations to our study. First, this retrospective study was conducted at a single referral center. Second, the administration of adjuvant therapy was decided case-by- case using multidisciplinary team approach, and was not based on the consistent guideline. Patients who expected to have relatively better outcomes were selected at the discretion of the treating physicians and surgeons, possibly biasing the outcome. Therefore, the adjuvant therapy group might have consisted of patients with relatively better performance status, which can influence OS. Therefore, our results should be interpreted carefully. We tried our best to improve internal validity, limiting the study to patient who completed the treatment in the same institution and only including cases who had undergone complete resection. However, surgical candidates with N2 disease are relatively rare and considering heterogeneity of N2 disease, it is difficult to conduct a well-designed randomized controlled trial. Our real-world practice provides some evidence for further investigations.
In conclusion, administration of adjuvant therapy following trimodal therapy was a significant prognostic indicator of OS and DFS. To confirm our results, better-designed prospective study is required.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.23). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was given approval by the Institutional Review Board of Samsung Medical Center (IRB No. 2015-05-143).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst 1994;86:673-80. [Crossref] [PubMed]
- Rosell R, Gomez-Codina J, Camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med 1994;330:153-8. [Crossref] [PubMed]
- Lorent N, De Leyn P, Lievens Y, et al. Long-term survival of surgically staged IIIA-N2 non-small-cell lung cancer treated with surgical combined modality approach: analysis of a 7-year prospective experience. Ann Oncol 2004;15:1645-53. [Crossref] [PubMed]
- Friedel G, Budach W, Dippon J, et al. Phase II trial of a trimodality regimen for stage III non-small-cell lung cancer using chemotherapy as induction treatment with concurrent hyperfractionated chemoradiation with carboplatin and paclitaxel followed by subsequent resection: a single-center study. J Clin Oncol 2010;28:942-8. [Crossref] [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]
- Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452-60. [Crossref] [PubMed]
- Eberhardt WE, De Ruysscher D, Weder W, et al. 2nd ESMO Consensus Conference in Lung Cancer: locally advanced stage III non-small-cell lung cancer. Ann Oncol 2015;26:1573-88. [Crossref] [PubMed]
- Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e314S-e40S.
- Tsuji M, Murota SI, Morita I. Docosapentaenoic acid (22:5, n-3) suppressed tube-forming activity in endothelial cells induced by vascular endothelial growth factor. Prostaglandins Leukot Essent Fatty Acids 2003;68:337-42. [Crossref] [PubMed]
- Nagai K, Tsuchiya R, Mori T, et al. A randomized trial comparing induction chemotherapy followed by surgery with surgery alone for patients with stage IIIA N2 non-small cell lung cancer (JCOG 9209). J Thorac Cardiovasc Surg 2003;125:254-60. [Crossref] [PubMed]
- Seder CW, Allen MS, Cassivi SD, et al. Stage IIIA non-small cell lung cancer: morbidity and mortality of three distinct multimodality regimens. Ann Thorac Surg 2013;95:1708-16. [Crossref] [PubMed]
- Betticher DC, Hsu Schmitz SF, Totsch M, et al. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: a multicenter phase II trial. J Clin Oncol 2003;21:1752-9. [Crossref] [PubMed]
- Betticher DC, Hsu Schmitz SF, Totsch M, et al. Prognostic factors affecting long-term outcomes in patients with resected stage IIIA pN2 non-small-cell lung cancer: 5-year follow-up of a phase II study. Br J Cancer 2006;94:1099-106. [Crossref] [PubMed]
- Decaluwé H, De Leyn P, Vansteenkiste J, et al. Surgical multimodality treatment for baseline resectable stage IIIA-N2 non-small cell lung cancer. Degree of mediastinal lymph node involvement and impact on survival. Eur J Cardiothorac Surg 2009;36:433-9. [Crossref] [PubMed]
- Paul S, Mirza F, Port JL, et al. Survival of patients with clinical stage IIIA non-small cell lung cancer after induction therapy: age, mediastinal downstaging, and extent of pulmonary resection as independent predictors. J Thorac Cardiovasc Surg 2011;141:48-58. [Crossref] [PubMed]
- Garrido P, Gonzalez-Larriba JL, Insa A, et al. Long-term survival associated with complete resection after induction chemotherapy in stage IIIA (N2) and IIIB (T4N0-1) non small-cell lung cancer patients: the Spanish Lung Cancer Group Trial 9901. J Clin Oncol 2007;25:4736-42. [Crossref] [PubMed]
- Yamane Y, Ishii G, Goto K, et al. A novel histopathological evaluation method predicting the outcome of non-small cell lung cancer treated by neoadjuvant therapy: the prognostic importance of the area of residual tumor. J Thorac Oncol 2010;5:49-55. [Crossref] [PubMed]
- Lee H, Ahn YC, Pyo H, et al. Pretreatment clinical mediastinal nodal bulk and extent do not influence survival in N2-positive stage IIIA non-small cell lung cancer patients treated with trimodality therapy. Ann Surg Oncol 2014;21:2083-90. [Crossref] [PubMed]
- Kim HK, Choi YS, Kim K, et al. Outcomes of mediastinoscopy and surgery with or without neoadjuvant therapy in patients with non-small cell lung cancer who are N2 negative on positron emission tomography and computed tomography. J Thorac Oncol 2011;6:336-42. [Crossref] [PubMed]
- Lim HJ, Joo S, Oh SH, et al. Syngeneic Myoblast Transplantation Improves Muscle Function in a Murine Model of X-Linked Myotubular Myopathy. Cell Transplant 2015;24:1887-900. [Crossref] [PubMed]
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60. [Crossref] [PubMed]
- Arriagada R, Dunant A, Pignon JP, et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol 2010;28:35-42. [Crossref] [PubMed]
- Arriagada R, Auperin A, Burdett S, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet 2010;375:1267-77. [Crossref] [PubMed]
- Lally BE, Zelterman D, Colasanto JM, et al. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol 2006;24:2998-3006. [Crossref] [PubMed]
- Douillard JY, Rosell R, De Lena M, et al. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: the adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. Int J Radiat Oncol Biol Phys 2008;72:695-701. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Jones VL, et al. Pulmonary resection after concurrent chemotherapy and high dose (60Gy) radiation for non-small cell lung cancer is safe and may provide increased survival. Eur J Cardiothorac Surg 2009;35:718-23; discussion 723. [Crossref] [PubMed]
- Thomas M, Rube C, Hoffknecht P, et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol 2008;9:636-48. [Crossref] [PubMed]
- van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 2007;99:442-50. [Crossref] [PubMed]