
Bullae formation hypothesis in primary spontaneous pneumothorax
Introduction
Primary spontaneous pneumothorax (PSP) typically occurs in tall and thin subjects without clinically apparent underlying lung disease (1). The incidence of PSP has geographical and gender-based differences. In the United States, 7.4 cases (age-adjusted incidence) per 100,000 population develop annually in males and 1.2 cases per 100,000 population per year in females (2). In the United Kingdom, 37 cases per 100,000 population develop annually in males and 15.4 cases per 100,000 population per year in females (3). The reasons for these differences are still unknown. The most common cause of a PSP is the rupture of small subpleural bleb/bullae (4). Subpleural bullae are found in most patients during thoracoscopy or thoracotomy (5). However, it remains uncertain how the bullae, the key of pathogenesis for PSP, have been formed in the young, healthy lungs.
We set out to classify the pattern of bullae formation for 458 PSP patients under 30 years of age who underwent thoracoscopic bullectomy from March 2004 to February 2012 at a single institution. Through preoperative chest computed tomography (CT; especially, coronal section) and intraoperative findings of PSP patients, we discovered three new findings that have been commonly seen but have not been given any meaning to them in the past. Furthermore, utilizing these findings, we hypothesized that the mechanism of bleb/bullae formation might be very closely related to the physical compression and friction effects of the surrounding chest wall structures on the lung parenchyma.
New terminology and the bullae formation hypothesis
First, note a “rib-line” that occurs in the shape of a band where the upper lobe of the lung is in contact with the ribs, mostly from the first to the fifth ribs (Figures 1,2). This rib-line is a mark on the lung surface resulting from mechanical compression and repeated friction by the ribs. As a result, the lung parenchyma may result in fibrosis through a chronic inflammatory reaction, or in bleb/bullae formation through emphysematous like changes. Previous studies for PSP etiology demonstrated the diffuse pleural porosity, inflammatory cell infiltration, fibrotic scar, and compensatory emphysema in the pathologic examination of the lung specimen (6). We found that the rib-line could explain all these changes. Pathologic results also add credibility to our hypothesis. Of course, the rib-line can be seen even in a healthy population, however, in a tall and thin person, the bony prominence of the ribs is more pronounced, and the narrow thoracic cavity makes the friction more pronounced between the lung and the ribs, resulting in more prominent rib-lines.
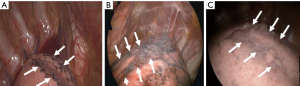
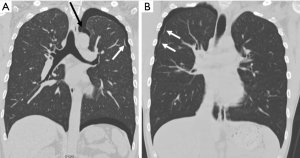
Secondly, we can see a small hole that penetrates the parietal pleura, which we have named “p-spot (pneumothorax-spot)”, which is more visible when the lung collapse occurs in PSP. It is an evidence that the small bleb from the lung apex has been located in the mediastinal soft tissue layer, digging into the chest wall that has been in contact for a long time (Figure 3). Preoperative CT findings of 458 PSP patients revealed that 98.9% (453/458) had bleb/bullae in the posterior part of lung apex, and 29.7% (136/458) had p-spots.
In 2014, Lin et al. first reported the presence of vascular-penetration defect (VPD) in 40.9% of PSP patients (7). The VPD they indicated is the same phenomenon as the p-spot we named. However, they explained with a different concept that the pulling of the visceral pleura by the negative pressure gradient in the lung apex produced VPD. As is well known, the chest cavity is a vacuum structure with negative pressure under normal breathing. We guess that initial bleb formation will be difficult in this normal and calm state of breathing. For bleb to be initially created, a strong pressure will be required to overcome the resistance of the visceral pleura to remain unchanged. We should pay attention to situations like Valsalva maneuver, including heavy lifting, forceful coughing, straining on the toilet, blowing a balloon, holding breath, or vomiting. It means a strong transient increase in intrathoracic pressure. Such situations are closer than we realize. This results in strong intrapulmonary positive pressure, inward movement of the chest wall, and diaphragm elevation. Lung and ribs are hit directly by positive pressure at this moment. This repetitive friction itself creates emphysematous like changes or bleb/bullae, while at the same time making the balloon effect in the apex of the upper lobe or superior segment of the lower lobe. Once the bleb is formed, intrathoracic negative pressure will be the basis for maintaining and developing its morphology. As the positive intrapulmonary pressure makes a bleb, it is assumed that the VPD can be made by the mechanical compression of the bleb. The stimulus during this process is thought to evoke angiogenesis. We still think that the term “p-spot” works better because this is very directly related to pneumothorax or hemopneumothorax.
If a bleb whose perimeter is all adhered to surrounding parietal pleura bursts into the mediastinal soft tissue, only primary spontaneous pneumomediastinum can occur without PSP. When the most vulnerable bulla ruptures and the lung collapse happens in succession, the detachment force from the parietal pleura rises to the bleb placed on the p-spot. If the force of this detachment damages the blood vessel in the area, bleeding may occur and become primary spontaneous hemopneumothorax.
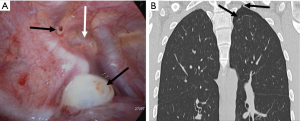
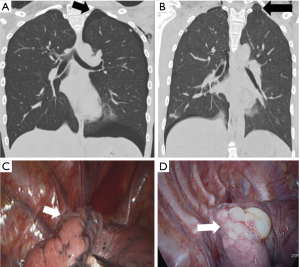
Thirdly, note a “strangle sign” shows a bulla captured and surrounded by adjacent thoracic structures in the apex of the thoracic cavity. We have observed that in many patients with PSP, the bullae were formed in the posterior portion of the lung apex (Figure 4). That region shows the balloon effect resulting from the influence of the compressed upper lobe by ribs, thus forming bullae (Figure 5). If the p-spot is a sign of a digging trace of a small bleb, the strangle sign is a squeezed bulla into the apico-posterior space surrounded by the 1st rib and subclavian vessels. Once a small bleb has settled in this apical space, it tends to take up more and more space. The surrounding structures intensify the choking of the bullae. Naturally, the rupture of the bullae also occurs most commonly at this site.
The implication of the hypothesis
There have been several bullae formation hypotheses and risk factor analyses for PSP occurrence including discrepancy between the static lung growth and the rapid increase in chest height (8), pleural porosity (9), FLCN (folliculin) gene mutation (10), distal airway inflammation (11), hereditary predisposition (12), abnormalities of the bronchial anatomy (13), ectomorphic physiognomy with more negative intrapleural pressures (14), and apical ischemia at the lung apex (15), low body mass index and caloric restriction (16). However, they were not enough to cover the overall pathogenesis of PSP patients. For example, the theory of apical lung ischemia is difficult to explain the bullae pattern on the lateral surface of the upper lobe. It accounts for 27.9% (128/458) in our PSP patients. Our hypothesis can help predict the location of the ruptured bullae and give a rationale that covering the lung apex with a bioabsorbable reinforcement sheet rather than mechanical pleurodesis may be more effective in preventing recurrence during PSP surgery (17). Also, it may be a good starting point to combine thoracic deformity correction with bullectomy operation in the case of PSP patients with a definite funnel chest.
Testing the hypothesis
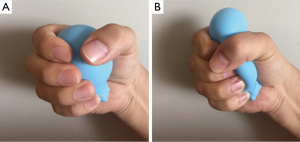
In our ex-vivo porcine lung experiment (18), we incidentally found that a bleb was able to be made by the friction of the lung with two fingers. However, an objective and quantified animal experiment model would be needed.
Under the assumption that bullae formation is related to the shape of the chest, we can infer the link with bullae formation by analyzing the patients with pectus excavatum, which is a typical thoracic deformity disease. Recent studies have shown that PSP patients have higher Haller index, anteroposteriorly flatter, laterally narrower, and craniocaudally taller thorax compared with age and sex-matched control population (19,20). Interestingly, pectus excavatum has male predominance with a similar pattern to the incidence of PSP (21).
Limitations
The incidences of the rib-line and strangle sign have not yet been investigated with data due to the limitations of the retrospective study. Intraoperative findings are essential for the confirmation of preoperative CT findings. Further research will be needed to compare preoperative CT and intraoperative findings in young generations with and without bullae.
Conclusions
We propose an association between narrow chest, mechanical compression/friction, rib-line, balloon effect, bullae formation, and PSP. Our model enables intuitive understanding through figures and helps us realize another dimension of PSP pathogenesis through new terminology. However, we still need more tests for the widely accepted theory.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.39). HCY serves as the unpaid editorial board member of Journal of Thoracic Disease from Feb 2019 to Jan 2021. SJ has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Noppen M. Spontaneous pneumothorax: epidemiology, pathophysiology and cause. Eur Respir Rev 2010;19:217-9. [Crossref] [PubMed]
- Melton LJ 3rd, Hepper NG, Offord KP. Incidence of spontaneous pneumothorax in Olmsted County, Minnesota: 1950 to 1974. Am Rev Respir Dis 1979;120:1379-82. [PubMed]
- Light RW. Pleural Diseases. 6th edition. Philadelphia: Lippincott Williams and Wilkins, 2013.
- Light RW. Management of spontaneous pneumothorax. Am Rev Respir Dis 1993;148:245-8. [Crossref] [PubMed]
- Sahn SA, Heffner JE. Spontaneous pneumothorax. N Engl J Med 2000;342:868-74. [Crossref] [PubMed]
- Grundy S, Bentley A, Tschopp JM. Primary Spontaneous Pneumothorax: A Diffuse Disease of the Pleura. Respiration 2012;83:185-9. [Crossref] [PubMed]
- Lin FC, Chou M, Jeng K, et al. Vascular-penetration defect detected in parietal pleura of primary spontaneous pneumothorax. Interact Cardiovasc Thorac Surg 2014;19:861-3. [Crossref] [PubMed]
- Chang PY, Wong KS, Lai JY, et al. Rapid increase in the height and width of the upper chest in adolescents with primary spontaneous pneumothorax. Pediatr Neonatol 2015;56:53-7. [Crossref] [PubMed]
- Radomsky J, Becker HP, Hartel W. Pleural porosity in idiopathic spontaneous pneumothorax. Pneumologie 1989;43:250-3. [PubMed]
- Graham RB, Nolasco M, Peterlin B, et al. Nonsense mutations in folliculin presenting as isolated familial spontaneous pneumothorax in adults. Am J Respir Crit Care Med 2005;172:39-44. [Crossref] [PubMed]
- Bense L, Lewander R, Eklund G, et al. Nonsmoking, non-alpha 1-antitrypsin deficiency-induced emphysema in nonsmokers with healed spontaneous pneumothorax, identified by computed tomography of the lungs. Chest 1993;103:433-8. [Crossref] [PubMed]
- Morrison PJ, Lowry RC, Nevin NC. Familial primary spontaneous pneumothorax consistent with true autosomal dominant inheritance. Thorax 1998;53:151-2. [Crossref] [PubMed]
- Bense L, Eklund G, Wiman LG. Bilateral bronchial anomaly. A pathogenetic factor in spontaneous pneumothorax. Am Rev Respir Dis 1992;146:513-6. [Crossref] [PubMed]
- Fujino S, Inoue S, Tezuka N, et al. Physical development of surgically treated patients with primary spontaneous pneumothorax. Chest 1999;116:899-902. [Crossref] [PubMed]
- Withers JN, Fishback ME, Kiehl PV, et al. Spontaneous pneumothorax. Suggested etiology and comparison of treatment methods. Am J Surg 1964;108:772-6. [Crossref] [PubMed]
- Coxson HO, Chan IHT, Mayo JR, et al. Early emphysema in patients with anorexia nervosa. Am J Respir Crit Care Med 2004;170:748-52. [Crossref] [PubMed]
- Hirai K, Kawashima T, Takeuchi S, et al. Covering the staple line with a polyglycolic acid sheet after bullectomy for primary spontaneous pneumothorax prevents postoperative recurrent pneumothorax. J Thorac Dis 2015;7:1978-85. [PubMed]
- Yang HC, Chang HY. Novel air leak test using surfactant for lung surgery. J Thorac Dis 2018;10:6472-4. [Crossref] [PubMed]
- Park CH, Sung MD, Lee GD, et al. Risk of Primary Spontaneous Pneumothorax According to Chest Configuration. Thorac cardiovasc Surg 2018;66:583-8. [Crossref] [PubMed]
- Saita K, Murakawa T, Kawano H, et al. Chest wall deformity found in patients with primary spontaneous pneumothorax. Asian Cardiovasc Thorac Ann 2013;21:582-7. [Crossref] [PubMed]
- Brochhausen C, Turial S, Müller FKP, et al. Pectus excavatum: history, hypotheses and treatment options. Interact Cardiovasc Thorac Surg 2012;14:801-6. [Crossref] [PubMed]


