Is needle biopsy a risk factor of pleural recurrence after surgery for non-small cell lung cancer?
Introduction
Preoperative computed tomography-guided needle biopsies (PCTGNBs) and intraoperative fine-needle aspiration biopsies (IFNABs) are valuable examinations for the evaluation of indeterminate pulmonary nodules with high accuracy and safety for the detection of malignancy (1-4), therefore they are important alternatives to trans-bronchial biopsies (TBBs) through bronchofiber scopes or surgical resection. However, several complications of PCTGNB and IFNAB have been reported, including tumor seeding and increasing the frequency of ipsilateral pleural recurrence (PR) (5-7). Although it is not conclusive whether such needle aspiration can be a potential hazard of PR, the two modalities are being applied in practice. On the other hand, PR with pleural dissemination or malignant effusion has sometimes been experienced in cases of pathologic stage IA lung cancer.
We conducted a retrospective study to identify potential risk factors of PR in patients with non-small cell lung cancer (NSCLC) after curative resection, especially focusing on whether PCTGNB and IFNAB are related to PR.
Methods
Patients
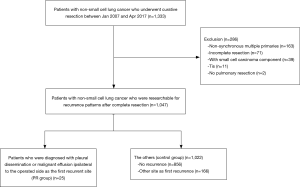
We performed a retrospective chart review of the 1,333 primary lung cancer patients who underwent curative lung resection at Chiba University Hospital between 2008 and 2017. Among them, patients with non-synchronous multiple primaries (n=163), incomplete resections (n=71), small cell lung cancer components (n=39), Tis (n=11), or no pulmonary resections (n=2) were excluded. The remaining 1,047 patients were divided into two groups: those who were diagnosed with pleural dissemination or malignant effusion ipsilateral to the operated side as the first recurrent site (PR group, n=25), and the others (control group, n=1,022), including patients without any recurrences (n=856) and those with recurrences other than PR (n=166) (Figure 1).
All the patients’ medical histories, surgical records, and radiological as well as pathological results were reviewed. The pathological diagnosis was determined according to the World Health Organization lung cancer classification. The staging was determined according to the 7th lung cancer TNM classification and staging system. The Ethics Committee at Chiba University approved this study (No. 3045) and written consent was waived due to the study being a retrospective chart review.
PCTGNB procedure
In principal, PCTGNB is applied to patients that have no indication or cannot be diagnosed with TBB. Briefly, a 16-gauge co-axial system needle was guided into the lesion though the intercostal space under real-time computed tomography (CT) imaging with local anesthesia. Fine-needle aspiration cytology with a 21- or 22-gauge single needle was occasionally used in patients who had emphysematous lungs or centrally located lesions to avoid complications, such as critical pneumothorax or pulmonary hemorrhage.
IFNAB procedure
IFNAB was applied to patients who had a preoperatively undiagnosed peripheral mass in the outer one-third of the lung parenchyma. At the time of surgery, the tumor was detected by ocular inspection or by palpation. Aspiration cytology was performed using a 22-gauge single needle attached to a syringe through the pulmonary parenchyma. This process was generally repeated twice to obtain an adequate specimen. The specimen was carried to cytologists/pathologists, subjected to Giemsa stain, and the results were conveyed to the operation room within 20 to 30 minutes. When the specimens were not sufficient to diagnose, surgical biopsy by wedge resection was performed. IFNAB was usually applied with electrocoagulation for hemostasis.
Follow-up strategy
All patients had undergone a curative resection, including systematic nodal dissection (the area of which was defined according to the criteria of the Japan Lung Cancer Society), and were subjected to the routine follow-up protocol in our outpatient clinic. The physical examination included: clinical blood tests, including blood cell counts, hepatic enzymes, creatinine, total protein and tumor markers (carcinoembryonic antigen, cytokeratin 19 fragments and pro-gastrin-releasing peptide); and chest X-rays, which were performed every 3 months for the first 2 years and every 6 months thereafter until 5 years after surgery. A thoraco-abdominal CT scan was routinely taken every 6 months, while a bone scan and brain magnetic resonance imaging were performed every 12 months during the follow-up period. After 5 years, an annual CT scan was optionally taken. If there were any subjective or objective symptoms, further relevant examinations were promptly performed. When recurrence was suspected based on imaging, a biopsy was performed for the diagnosis of recurrence if possible.
Assessment of PR
PR was defined as the development of a pleural lesion or malignant effusion, or both, in the hemithorax of the operated side at the first relapse. Malignant pleural effusion was diagnosed cytologically, and pleural dissemination was diagnosed when multiple enhanced pleural lesions were observed on the chest CT. Positron emission tomographic-CT was used in several recent cases to support the diagnosis.
Statistical analysis
Data are presented as mean ± SD. Student’s t test was used for continuous variables, and Pearson’s chi-squared test was used for categorical variables. A logistic regression model was used to calculate the probability of clinicopathological variables. Cox regression analysis was performed to identify the risk factors associated with PR after surgery. Actuarial recurrence curves were calculated by the Kaplan-Meier method. Differences in recurrence rate were assessed by the log-rank test. All tests were two-sided and a P value of <0.05 was used to define statistical significance. Statistical analyses were performed using JMP (SAS, Cary, NC, USA).
Results
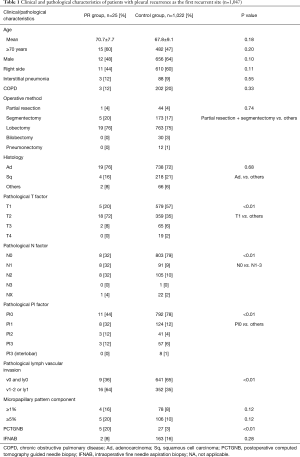
Of the 1,047 patients, recurrence occurred in 191 patients (18%), and 25 patients (2%) were in the PR group (pleural dissemination 8, pleural effusion 15, both dissemination and effusion 2). The follow-up periods in the two groups were comparable, with a median of 23.0 months in the PR group and 37.0 months in the control group. The clinicopathologic characteristics are summarized in Table 1. There was no significant difference in age, sex, the presence of chronic obstructive pulmonary disease or interstitial pneumonia, type of operative methods, histological types and the presence of micropapillary pattern between the two groups. Regarding the pathological findings of the tumor, tumor size was larger and lymph node metastasis was more common in the PR group than in the control group. In terms of diagnostic procedures, 5 of 25 patients (20%) in the PR group and 27 of 1,022 patients (3%) in the control group underwent PCTGNB, and there was a significant difference between the two groups (P<0.01). In contrast, the proportion of patients who underwent IFNAB was not significantly different between the two groups.
Full table
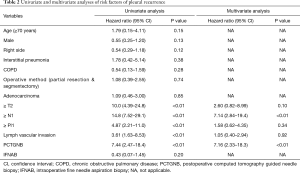
To determine the risk factors for PR, univariate and multivariate Cox regression analyses were performed (Table 2). The univariate analysis identified pathological tumor (T), lymph nodes (N), pleural invasion (Pl), lymphatic invasion (ly) and vascular invasion (v) factors and PCTGNB as risk factors for PR (P<0.01). Among them, the hazard ratio (HR) of pathological N factor was the highest at 14.8 [95% confidence interval (CI), 7.52–29.1] of all factors. The HR of PCTGNB was 7.44 (95% CI, 2.47–18.4); however, IFNAB was not identified as a risk factor (HR, 0.43; 95% CI, 0.07–1.45). A multivariate analysis revealed pathological N factor and the frequency of PCTGNB as independent risk factors for PR, with HRs of 7.14 (95% CI, 2.84–19.4; P<0.01) and 7.16 (95% CI, 2.33–18.3; P<0.01), respectively.
Full table
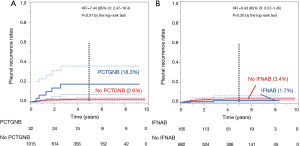
The 5-year PR rate of patients who underwent PCTGNB (n=32) was higher at 18.3% than that of the others at 2.6% (n=1,015, P<0.01 by the log-rank test, Figure 2A). On the other hand, the 5-year PR rate of the IFNAB group (n=165) was 1.7% and that of the others (n=882) was 3.4%; there was no significant difference (P=0.20) (Figure 2B).
The details of 5 patients with PR after surgery and who underwent PCTGNB are individually summarized in Table 3. Pathological N2 was observed in 2 patients and pathological Pl2 or 3 was observed in another 2 patients. However, a case of early-stage NSCLC was found. The tumor had a very low risk of recurrence because it was equivalent to a minimally invasive adenocarcinoma in the 8th lung cancer TNM classification. Therefore, it was strongly suspected that tumor seeding really occurred and was associated with PR in that case.

Full table
Discussion
This study analyzed risk factors for PR, especially focusing on diagnostic needle biopsies such as PCTGNB and IFNAB in patients with lung cancer who underwent curative resection. When it comes to recurrence in the pleural cavity, even in early disease in surgical pathology, patients generally have a poor prognosis and are considered unsuitable candidates for local therapy (8). Moreover, treatment and the control of malignant pleural effusions sometimes become extremely difficult (9). Therefore, it is very important to identify risk factors of PR and to prevent tumor seeding by preoperative diagnosis or operative procedures.
Although PCTGNB is widely used for histological diagnosis because of its high accuracy at 92.1% (1), several complications associated with this examination have been reported (5-7). According to a questionnaire survey, the most frequent complication is pneumothorax with a reported rate of approximately 20.5–35%, followed by hemoptysis (5.3%), hemothorax (0.09%), air embolism (0.06%) and tumor seeding (0.01–0.06%) (6,10,11). However, the true incidence of tumor seeding along the needle tract may be underestimated because not all cases are fully followed-up. Therefore, it is necessary to follow patients for several years to clinically identify dissemination after the procedure. Several reports have indicated that PCTGNB influences PR in completely resected lung cancer (12,13) but other studies have shown conflicting results (14-17). A recent meta-analysis concluded that PCTGNB does not increase the risk of PR; however, a subgroup analysis in that study showed that PCTGNB is associated with increased PR in cases with sub-pleural lesions (18). This is likely inconclusive because the meta-analysis was based on only 5 single-center retrospective studies with small samples. In addition, different characteristics, procedure criteria and puncture methods could be also confounding factors and might affect the outcome. Another study using the inverse probability-weighted model to adjust for characteristics demonstrated that PCTGNB was an independent risk factor of PR (19). We conducted a multivariate analysis to minimize the influences of confounding factors like patients’ characteristics and pathological factors and found that PCTGNB was an independent risk factor of PR after lung resection. Therefore, we propose that PCTGNB should be limited to cases that absolutely require a pathological diagnosis for deciding treatment strategy.
IFNAB can minimize the time until radical surgery for peripheral lung cancer by avoiding further examinations and is the simplest and most direct method for diagnosing lung tumors (3). This examination is considered to increase the potential risk of PR through needle tracts like PCTGNB, though there is no clear evidence in this study. An experimental study demonstrated a significantly higher pleural dissemination potential by performing pleural washing and cytology on resected lungs before and after IFNAB. Cytology positive for malignancy was found in 60% of the post-IFNAB specimens compared with 10% of the pre-IFNAB specimens (20). However, a single-center retrospective study indicated that IFNAB did not increase the risk of pleural dissemination (3). In our study, there were only 2 patients (8% of IFNAB) who experienced PR after surgery with IFNAB and there was no relationship between IFNAB and PR. The 2 patients had other risk factors for PR such as N1 and N2. It is also evident that seeded cancer cells cannot always generate pleural dissemination, as it probably requires several other factors, such as adhesion, vascular generation, and transformation (3). Disseminated cancer cells at the time of IFNAB might be washed out by irrigation with warm saline just before closure of the thorax.
Lymph node metastasis is known to be one of the most powerful factors leading to a poor prognosis in patients with completely resected cancer due to high local and distant recurrence rates (21,22). Our results indicate that the existence of metastatic lymph nodes clearly influences PR. Possible mechanisms to explain this are that the cancer cells metastasizing to the lymph nodes were exposed to the pleural cavity or were blocking lymphatic drainage from the lung parenchyma, or that the metastatic nodes were destroyed during surgery, leading to the direct seeding of cancer cells. Although further investigation is required to prove these mechanisms, it is important information to surgeons.
Relevant limitations of this monocentric study are the retrospective design of the data and the relatively small number of patients. Patients who were diagnosed with pleural disease at the time of thoracotomy were omitted from this study because it is difficult to distinguish natural pleural disease and artificial pleural dissemination. During the study period, malignant pleurisy and intrathoracic dissemination were first found during surgery in 21 patients, 2 of which had undergone PCTGNB. Another limitation may be a potential selection bias. PCTGNB might have been applied to peripherally located tumors. Further investigation would be required to provide robust evidence on the potential impact of these practical issues.
In conclusion, although IFNAB was not a risk factor of postoperative PR, the PCTGNB procedure is strongly associated with the occurrence of PR after curative resection in this study. As there is not only the risk of tumor seeding, but also the risk of the other complications like pneumothorax, indications for PCTGNB should be carefully considered for the candidates of NCSLC resection. Alternatively, IFNAB or intraoperative rapid diagnosis of frozen sections acquired from a minimal surgical resection is more recommended for patients whose pulmonary lesion is suspicious of lung cancer but not diagnosed cytologically or pathologically.
Acknowledgments
This paper was presented in the 26th European Conference on General Thoracic Surgery, May 27-30, 2018, Ljubljana, Slovenia.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.16). YS serves as an unpaid editorial board member of Journal of Thoracic Disease from Sep 2018 to Aug 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Ethics Committee at Chiba University approved this study (No. 3045) and written consent was waived due to the study being a retrospective chart review.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- DiBardino DM, Yarmus LB, Semaan RW. Transthoracic needle biopsy of the lung. J Thorac Dis 2015;7:S304-16. [PubMed]
- Winokur RS, Pua BB, Sullivan BW, et al. Percutaneous lung biopsy: technique, efficacy, and complications. Semin Intervent Radiol 2013;30:121-7. [Crossref] [PubMed]
- Matsuoka T, Sonobe M, Date H. Intraoperative fine-needle aspiration biopsy (FNA) for lung cancer: diagnostic value and risk of pleural dissemination. Surg Today 2015;45:695-9. [Crossref] [PubMed]
- Biancosino C, Kruger M, Vollmer E, et al. Intraoperative fine needle aspirations - diagnosis and typing of lung cancer in small biopsies: challenges and limitations. Diagn Pathol 2016;11:59. [Crossref] [PubMed]
- Murphy JM, Gleeson FV, Flower CD. Percutaneous needle biopsy of the lung and its impact on patient management. World J Surg 2001;25:373-9; discussion 379-80. [Crossref] [PubMed]
- Tomiyama N, Yasuhara Y, Nakajima Y, et al. CT-guided needle biopsy of lung lesions: a survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol 2006;59:60-4. [Crossref] [PubMed]
- Ibukuro K, Tanaka R, Takeguchi T, et al. Air embolism and needle track implantation complicating CT-guided percutaneous thoracic biopsy: single-institution experience. AJR Am J Roentgenol 2009;193:W430-6. [Crossref] [PubMed]
- Mizuno T, Arimura T, Kuroda H, et al. Current outcomes of postrecurrence survival in patients after resection of non-small cell lung cancer. J Thorac Dis 2018;10:1788-96. [Crossref] [PubMed]
- Penz E, Watt KN, Hergott CA, et al. Management of malignant pleural effusion: challenges and solutions. Cancer Manag Res 2017;9:229-41. [Crossref] [PubMed]
- Ayar D, Golla B, Lee JY, et al. Needle-track metastasis after transthoracic needle biopsy. J Thorac Imaging 1998;13:2-6. [Crossref] [PubMed]
- Richardson CM, Pointon KS, Manhire AR, et al. Percutaneous lung biopsies: a survey of UK practice based on 5444 biopsies. Br J Radiol 2002;75:731-5. [Crossref] [PubMed]
- Inoue M, Honda O, Tomiyama N, et al. Risk of pleural recurrence after computed tomographic-guided percutaneous needle biopsy in stage I lung cancer patients. Ann Thorac Surg 2011;91:1066-71. [Crossref] [PubMed]
- Matsuguma H, Nakahara R, Kondo T, et al. Risk of pleural recurrence after needle biopsy in patients with resected early stage lung cancer. Ann Thorac Surg 2005;80:2026-31. [Crossref] [PubMed]
- Asakura K, Izumi Y, Yamauchi Y, et al. Incidence of pleural recurrence after computed tomography-guided needle biopsy in stage I lung cancer. PLoS One 2012;7:e42043. [Crossref] [PubMed]
- Flechsig P, Kunz J, Heussel CP, et al. Invasive lung cancer staging: influence of CT-guided core needle biopsy on onset of pleural carcinomatosis. Clin Imaging 2015;39:56-61. [Crossref] [PubMed]
- Kashiwabara K, Semba H, Fujii S, et al. Preoperative Percutaneous Transthoracic Needle Biopsy Increased the Risk of Pleural Recurrence in Pathological Stage I Lung Cancer Patients With Sub-pleural Pure Solid Nodules. Cancer Invest 2016;34:373-7. [Crossref] [PubMed]
- Ahn SY, Yoon SH, Yang BR, et al. Risk of pleural recurrence after percutaneous transthoracic needle biopsy in stage I non-small-cell lung cancer. Eur Radiol 2019;29:270-8. [Crossref] [PubMed]
- Wang T, Luo L, Zhou Q. Risk of Pleural Recurrence in Early Stage Lung Cancer Patients after Percutaneous Transthoracic Needle Biopsy: A Meta-analysis. Sci Rep 2017;7:42762. [Crossref] [PubMed]
- Moon SM, Lee DG, Hwang NY, et al. Ipsilateral pleural recurrence after diagnostic transthoracic needle biopsy in pathological stage I lung cancer patients who underwent curative resection. Lung Cancer 2017;111:69-74. [Crossref] [PubMed]
- Sawabata N, Ohta M, Maeda H. Fine-needle aspiration cytologic technique for lung cancer has a high potential of malignant cell spread through the tract. Chest 2000;118:936-9. [Crossref] [PubMed]
- Varlotto JM, Yao AN, DeCamp MM, et al. Nodal stage of surgically resected non-small cell lung cancer and its effect on recurrence patterns and overall survival. Int J Radiat Oncol Biol Phys 2015;91:765-73. [Crossref] [PubMed]
- Isaka M, Kojima H, Takahashi S, et al. Risk factors for local recurrence after lobectomy and lymph node dissection in patients with non-small cell lung cancer: Implications for adjuvant therapy. Lung Cancer 2018;115:28-33. [Crossref] [PubMed]