Video-assisted thoracic surgery and pneumothorax
Introduction
In 1937, Sattler was the first to identify bullae in a patient with spontaneous pneumothorax with the use of thoracoscopy (1). In the past decades, technological advances in microcameras, lenses, digital technology and monitors, as well as the use of general anaesthetic technique to allow one-lung ventilation, preceded the advent of video-assisted thoracic surgery (VATS). Nowadays most thoracic surgeons believe that VATS is the approach of choice when an interventional procedure is considered necessary for the treatment of pneumothorax (2). In addition to simple observation, provided by thoracoscopy, VATS enables the safe and efficient use of procedures necessary for the treatment of pneumothorax like mechanical pleurodesis, pleurectomy and bullectomy. Conservative management of spontaneous pneumothorax (observation, needle aspiration, chest tube, etc.) has proved to result in significant recurrence of 16-52%. The rate of recurrence increases dramatically with more episodes of spontaneous pneumothorax. With the lower morbidity of VATS, the accepted surgical indications for pneumothorax include (1,3): (I) persistent air leak (4); (II) recurrence; (III) radiologically demonstrated large bullae; (IV) spontaneous haemopneumothorax; (V) incomplete expansion of the lung (despite chest drainage and suction); (VI) tension pneumothorax; (VII) bilateral involvement; (VIII) spontaneous pneumothorax in a high- risk occupation (e.g., pilot); (IX) suspicion of malignancy.
There is some controversy among surgeons whether VATS is recommended for uncomplicated first time pneumothorax, although the technique is considered an acceptable treatment for such cases (3). The benefits of VATS procedure in relation to the classic posterolateral thoracotomy (3,5) are as following: (I) safe method for the elderly (>75 years) with low postoperative pulmonary complications; (II) less access trauma; (III) no rib spreading; (IV) quicker recovery; (V) lower hospitalization time; (VI) less postoperative parental narcotics and pain medication; (VII) postoperative stress markers are lower in VATS compared to open procedures; (VIII) earlier mobilization than open thoracotomy; (IX) less shoulder mobility dysfunction in the early postoperative period when compared with open thoracotomy (Figure 1).
Patient positioning
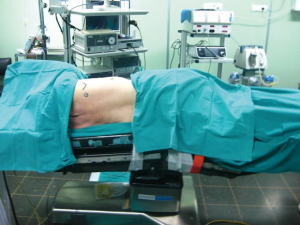
The patient is positioned in the lateral decubitus position with the operating table flexed at 30° to open up the intercostal spaces.
Incision and port placement
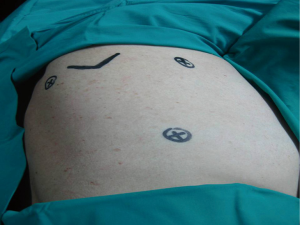
The standard VATS pleurodesis procedure requires three ports placed at the 7th intercostal space mid-axillary line, 4th intercostal space anterior axillary line and the last port just anterior to the tip of the scapula. The triangular port placement permits a panoramic view of the pleural cavity. The instruments and the camera ports should be sufficiently apart in a “triangulation” manner to prevent instrument collisions. Different technique, such as two hole or one hole technique, smaller telescopes, angled lenses to avoid torquing of the thoracoscope, avoiding the use of trocar ports for the insertion of instruments, have been developed and performed in various centers (Figure 2).
Procedure
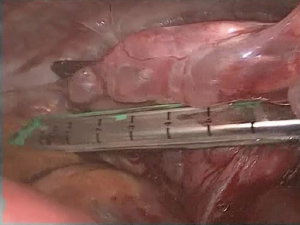
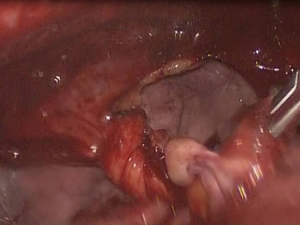
Inspection of the entire pleural cavity is required. Manipulation of the lung tissue is achieved with atraumatic blunt instruments (forceps). Adhesions should all be taken down to ensure good operating field using endoscopic instruments. Haemostasis is achieved with diathermy, staplers and endoclips. The division of apical adhesions asks for special attention due to their proximity to the subclavian vessels. The whole lung surface should be examined thoroughly for bullae due to the collapse of the lung which makes ruptured bullae easy to miss (Figure 3).
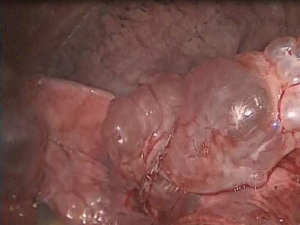
Endoscopic bullectomy is achieved with the help of various endo-staplers (6,7). The resection line should be across healthy lung tissue and crossing of staples should be avoided due to air leakage. In case of absence of visible bullae, stapling of the apical abnormality is always necessary. Caution needs to be applied to the traction of the lung during the bullectomy to prevent tearing of the organ. The resected segment can be retrieved through the port with the help of an endobag (Figure 4).
Mechanical pleurodesis is performed by using dry gauze or a mesh mounted at the end of an endoscopic instrument for abrasion (8,9). Pleurectomy may or may not be performed depending on the patient (10). Particular attention should be paid to ensure that mechanical pleurodesis is thoroughly performed at the apical and lateral regions of the pleural cavity, as well as the diaphragmatic surface—extra care is needed in the apex of the pleural cavity to avoid injury of the subclavian vessels. In young patients pleurectomy and pleurodesis are always considered in order to preserve the area near the mammary arteries for future use in case of coronary disease (Figure 5).
Different forms of pleural agents can be used for pleurodesis (6,10-20) such as: (I) talc slurry; (II) talc aerosol; (III) diluted fibrin glue; (IV) electrical brush system.
After careful haemostasis is achieved and no air leak from the staple line is observed, one or two chest tubes are inserted in the pleural cavity through the ports. The tube is directed to the apex and lie antero-laterally in the pleural cavity. The lung is reinflated under direct vision and the open wounds are closed (21-30).
Other techniques for managing bullae with VATS are (1): (I) Endoloop Ligation; (II) Endoscopic Suturing, which shows results similar to the endoscopic stapling and can be a viable solution in cases of small localized bullae; (III) Argon beam coagulator, which, according to the literature, is less effective than other techniques due to prolonged air leaks and higher rate of recurrence; (IV) uniportal VATS technique; (V) Two hole VATS technique (31-40). Except from the general contraindications like M.I., severe coagulopathy, inability to achieve one lung ventilation, ventilator dependency, other contraindications may include: (I) Severe underlying lung disease or poor lung function (inability to maintain satisfying ventilation with only one lung); (II) difficult adhesions—dense pleural symphysis (may be more suitable for open thoracotomy). As far as the complications of the procedure we may mark the following (41): (I) air leakage from the staple line; (II) wound infection; (III) bleeding; (IV) intercostal neuralgia; (V) surgical emphysema; (VI) empyema; (VII) complication of the pleurodesis agents.
A conversion to thoracotomy from VATS is required in 2-10% of the patients and is associated with technical difficulties and the experience of the surgeon (38,42-55).
The recurrence of pneumothorax after VATS treatment is reported to be slightly higher (2-14%) compared to that after open thoracotomy (0-7%), although this difference is constantly getting smaller (3,56,57). This may reflect the inadequate exposure of the chest cavity in VATS and subsequent incomplete detection and resection of all bullae. Thorough pleural examination and experience of the surgeon seem to be the two most crucial factors that determine recurrence (3,5).
With the technological advancements in modern surgical instruments, less minimally invasive techniques are rising, like Uniportal VATS, in which both the camera and the instruments are introduced to the thoracic cavity through a single port (58,59).
Although VATS is a more expensive procedure for treating pneumothorax than other alternative ways, financial planning at hospitals have achieved to lessen its cost to a degree similar to the cost of open thoracotomy or thoracoscopy (60). The smaller number of hospitalization days, and the quicker rehabilitation time of VATS treated patients prove that the procedure is cost-effective enough and compensate for the initial high cost.
In conclusion, stapled bullectomy, when followed by pleurodesis is a safe and reliable method for treating pneumothorax, with a low recurrence rate (5,61-64). Thorough pleural abrasion remains the key for reducing pneumothorax recurrence (1). Apical pleurectomy may further decrease the recurrence rate.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ng CS, Rocco G, Yim AP. Video-assisted thoracoscopic surgery (VATS) pleurodesis for pneumothorax. Multimed Man Cardiothorac Surg 2005;2005:mmcts.2004.000349.
- Yim AP, Ng CS. Thoracoscopy in the management of pneumothorax. Curr Opin Pulm Med 2001;7:210-4. [PubMed]
- Ng CS, Lee TW, Wan S, et al. Video assisted thoracic surgery in the management of spontaneous pneumothorax: the current status. Postgrad Med J 2006;82:179-85. [PubMed]
- Haga T, Kurihara M, Kataoka H. Spontaneous pneumothorax with persistent air leakage and invasive procedures. Intern Med 2013;52:2189-92. [PubMed]
- Fatimi SH, Hanif HM, Aziz S, et al. How VATS has changed the management of spontaneous pneumothorax in the 21st century. J Pak Med Assoc 2012;62:1041-5. [PubMed]
- Horio H, Nomori H, Kobayashi R, et al. Impact of additional pleurodesis in video-assisted thoracoscopic bullectomy for primary spontaneous pneumothorax. Surg Endosc 2002;16:630-4. [PubMed]
- Naunheim KS, Mack MJ, Hazelrigg SR, et al. Safety and efficacy of video-assisted thoracic surgical techniques for the treatment of spontaneous pneumothorax. J Thorac Cardiovasc Surg 1995;109:1198-203; discussion 1203-4. [PubMed]
- Gossot D, Galetta D, Stern JB, et al. Results of thoracoscopic pleural abrasion for primary spontaneous pneumothorax. Surg Endosc 2004;18:466-71. [PubMed]
- Passlick B, Born C, Häussinger K, et al. Efficiency of video-assisted thoracic surgery for primary and secondary spontaneous pneumothorax. Ann Thorac Surg 1998;65:324-7. [PubMed]
- Mouroux J, Elkaïm D, Padovani B, et al. Video-assisted thoracoscopic treatment of spontaneous pneumothorax: technique and results of one hundred cases. J Thorac Cardiovasc Surg 1996;112:385-91. [PubMed]
- Kioumis IP, Zarogoulidis K, Huang H, et al. Pneumothorax in cystic fibrosis. J Thorac Dis 2014;6:S480-7. [PubMed]
- Kuhajda I, Zarogoulidis K, Kougioumtzi I, et al. Tube thoracostomy; chest tube implantation and follow up. J Thorac Dis 2014;6:S470-9. [PubMed]
- Manika K, Kioumis I, Zarogoulidis K, et al. Pneumothorax in sarcoidosis. J Thorac Dis 2014;6:S466-9. [PubMed]
- Kuhajda I, Zarogoulidis K, Kougioumtzi I, et al. Penetrating trauma. J Thorac Dis 2014;6:S461-5. [PubMed]
- Visouli AN, Zarogoulidis K, Kougioumtzi I, et al. Catamenial pneumothorax. J Thorac Dis 2014;6:S448-60. [PubMed]
- Huang Y, Huang H, Li Q, et al. Transbronchial lung biopsy and pneumothorax. J Thorac Dis 2014;6:S443-7. [PubMed]
- Terzi E, Zarogoulidis K, Kougioumtzi I, et al. Acute respiratory distress syndrome and pneumothorax. J Thorac Dis 2014;6:S435-42. [PubMed]
- Boskovic T, Stojanovic M, Stanic J, et al. Pneumothorax after transbronchial needle biopsy. J Thorac Dis 2014;6:S427-34. [PubMed]
- Li Z, Huang H, Li Q, et al. Pneumothorax: observation. J Thorac Dis 2014;6:S421-6. [PubMed]
- Huang Y, Huang H, Li Q, et al. Approach of the treatment for pneumothorax. J Thorac Dis 2014;6:S416-20. [PubMed]
- Browning RF, Parrish S, Sarkar S, et al. Bronchoscopic interventions for severe COPD. J Thorac Dis 2014;6:S407-15. [PubMed]
- Machairiotis N, Kougioumtzi I, Dryllis G, et al. Laparoscopy induced pneumothorax. J Thorac Dis 2014;6:S404-6. [PubMed]
- Ouellette DR, Parrish S, Browning RF, et al. Unusual causes of pneumothorax. J Thorac Dis 2014;6:S392-403. [PubMed]
- Parrish S, Browning RF, Turner JF Jr, et al. The role for medical thoracoscopy in pneumothorax. J Thorac Dis 2014;6:S383-91. [PubMed]
- Terzi E, Zarogoulidis K, Kougioumtzi I, et al. Human immunodeficiency virus infection and pneumothorax. J Thorac Dis 2014;6:S377-82. [PubMed]
- Zarogoulidis P, Kioumis I, Pitsiou G, et al. Pneumothorax: from definition to diagnosis and treatment. J Thorac Dis 2014;6:S372-6. [PubMed]
- Tsakiridis K, Mpakas A, Kesisis G, et al. Lung inflammatory response syndrome after cardiac-operations and treatment of lornoxicam. J Thorac Dis 2014;6 Suppl 1:S78-98. [PubMed]
- Tsakiridis K, Zarogoulidis P, Vretzkakis G, et al. Effect of lornoxicam in lung inflammatory response syndrome after operations for cardiac surgery with cardiopulmonary bypass. J Thorac Dis 2014;6 Suppl 1:S7-20. [PubMed]
- Argiriou M, Kolokotron SM, Sakellaridis T, et al. Right heart failure post left ventricular assist device implantation. J Thorac Dis 2014;6 Suppl 1:S52-9. [PubMed]
- Madesis A, Tsakiridis K, Zarogoulidis P, et al. Review of mitral valve insufficiency: repair or replacement. J Thorac Dis 2014;6 Suppl 1:S39-51. [PubMed]
- Siminelakis S, Kakourou A, Batistatou A, et al. Thirteen years follow-up of heart myxoma operated patients: what is the appropriate surgical technique? J Thorac Dis 2014;6 Suppl 1:S32-8. [PubMed]
- Foroulis CN, Kleontas A, Karatzopoulos A, et al. Early reoperation performed for the management of complications in patients undergoing general thoracic surgical procedures. J Thorac Dis 2014;6 Suppl 1:S21-31. [PubMed]
- Nikolaos P, Vasilios L, Efstratios K, et al. Therapeutic modalities for Pancoast tumors. J Thorac Dis 2014;6 Suppl 1:S180-93. [PubMed]
- Koutentakis M, Siminelakis S, Korantzopoulos P, et al. Surgical management of cardiac implantable electronic device infections. J Thorac Dis 2014;6 Suppl 1:S173-9. [PubMed]
- Spyratos D, Zarogoulidis P, Porpodis K, et al. Preoperative evaluation for lung cancer resection. J Thorac Dis 2014;6 Suppl 1:S162-6. [PubMed]
- Porpodis K, Zarogoulidis P, Spyratos D, et al. Pneumothorax and asthma. J Thorac Dis 2014;6 Suppl 1:S152-61. [PubMed]
- Panagopoulos N, Leivaditis V, Koletsis E, et al. Pancoast tumors: characteristics and preoperative assessment. J Thorac Dis 2014;6 Suppl 1:S108-15. [PubMed]
- Visouli AN, Darwiche K, Mpakas A, et al. Catamenial pneumothorax: a rare entity? Report of 5 cases and review of the literature. J Thorac Dis 2012;4 Suppl 1:17-31. [PubMed]
- Zarogoulidis P, Chatzaki E, Hohenforst-Schmidt W, et al. Management of malignant pleural effusion by suicide gene therapy in advanced stage lung cancer: a case series and literature review. Cancer Gene Ther 2012;19:593-600. [PubMed]
- Papaioannou M, Pitsiou G, Manika K, et al. COPD assessment test: a simple tool to evaluate disease severity and response to treatment. COPD 2014;11:489-95. [PubMed]
- Yim AP, Liu HP. Complications and failures of video-assisted thoracic surgery: experience from two centers in Asia. Ann Thorac Surg 1996;61:538-41. [PubMed]
- Boskovic T, Stanic J, Pena-Karan S, et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis 2014;6 Suppl 1:S99-107. [PubMed]
- Papaiwannou A, Zarogoulidis P, Porpodis K, et al. Asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): current literature review. J Thorac Dis 2014;6 Suppl 1:S146-51. [PubMed]
- Zarogoulidis P, Porpodis K, Kioumis I, et al. Experimentation with inhaled bronchodilators and corticosteroids. Int J Pharm 2014;461:411-8. [PubMed]
- Bai C, Huang H, Yao X, et al. Application of flexible bronchoscopy in inhalation lung injury. Diagn Pathol 2013;8:174. [PubMed]
- Zarogoulidis P, Kioumis I, Porpodis K, et al. Clinical experimentation with aerosol antibiotics: current and future methods of administration. Drug Des Devel Ther 2013;7:1115-34. [PubMed]
- Zarogoulidis P, Pataka A, Terzi E, et al. Intensive care unit and lung cancer: when should we intubate? J Thorac Dis 2013;5 Suppl 4:S407-12. [PubMed]
- Hohenforst-Schmidt W, Petermann A, Visouli A, et al. Successful application of extracorporeal membrane oxygenation due to pulmonary hemorrhage secondary to granulomatosis with polyangiitis. Drug Des Devel Ther 2013;7:627-33. [PubMed]
- Zarogoulidis P, Kontakiotis T, Tsakiridis K, et al. Difficult airway and difficult intubation in postintubation tracheal stenosis: a case report and literature review. Ther Clin Risk Manag 2012;8:279-86. [PubMed]
- Zarogoulidis P, Tsakiridis K, Kioumis I, et al. Cardiothoracic diseases: basic treatment. J Thorac Dis 2014;6 Suppl 1:S1. [PubMed]
- Kolettas A, Grosomanidis V, Kolettas V, et al. Influence of apnoeic oxygenation in respiratory and circulatory system under general anaesthesia. J Thorac Dis 2014;6 Suppl 1:S116-45. [PubMed]
- Turner JF, Quan W, Zarogoulidis P, et al. A case of pulmonary infiltrates in a patient with colon carcinoma. Case Rep Oncol 2014;7:39-42. [PubMed]
- Machairiotis N, Stylianaki A, Dryllis G, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol 2013;8:194. [PubMed]
- Tsakiridis K, Zarogoulidis P. An interview between a pulmonologist and a thoracic surgeon-Pleuroscopy: the reappearance of an old definition. J Thorac Dis 2013;5 Suppl 4:S449-51. [PubMed]
- Huang H, Li C, Zarogoulidis P, et al. Endometriosis of the lung: report of a case and literature review. Eur J Med Res 2013;18:13. [PubMed]
- Waller DA, Forty J, Morritt GN. Video-assisted thoracoscopic surgery versus thoracotomy for spontaneous pneumothorax. Ann Thorac Surg 1994;58:372-6; discussion 376-7. [PubMed]
- Cole FH Jr, Cole FH, Khandekar A, et al. Video-assisted thoracic surgery: primary therapy for spontaneous pneumothorax? Ann Thorac Surg 1995;60:931-3; discussion 934-5. [PubMed]
- Salati M, Brunelli A. Uniportal VATS for pneumothorax and interstitial lung disease. J Thorac Dis 2013;5 Suppl 3:S217-20. [PubMed]
- Onodera K, Noda M, Okada Y, et al. Awake video-thoracoscopic surgery for intractable pneumothorax in pregnancy by using a single portal plus puncture. Interact Cardiovasc Thorac Surg 2013;17:438-40. [PubMed]
- Tschopp JM, Rami-Porta R, Noppen M, et al. Management of spontaneous pneumothorax: state of the art. Eur Respir J 2006;28:637-50. [PubMed]
- Vohra HA, Adamson L, Weeden DF. Does video-assisted thoracoscopic pleurectomy result in better outcomes than open pleurectomy for primary spontaneous pneumothorax? Interact Cardiovasc Thorac Surg 2008;7:673-7. [PubMed]
- Cardillo G, Facciolo F, Giunti R, et al. Videothoracoscopic treatment of primary spontaneous pneumothorax: a 6-year experience. Ann Thorac Surg 2000;69:357-61; discussion 361-2. [PubMed]
- Ayed AK, Al-Din HJ. The results of thoracoscopic surgery for primary spontaneous pneumothorax. Chest 2000;118:235-8. [PubMed]
- Sawada S, Watanabe Y, Moriyama S. Video-assisted thoracoscopic surgery for primary spontaneous pneumothorax: evaluation of indications and long-term outcome compared with conservative treatment and open thoracotomy. Chest 2005;127:2226-30. [PubMed]