Postoperative pain management
Introduction
Thoracic surgery can cause severe postoperative pain that can interfere adversely with the respiratory function, the performance, the outcome and the quality of life of patients. For that reasons, the post thoracotomy patient requires dynamic analgesia, which means that the patient will be able easily to cough, respirate deeply and move, in order to minimize respiratory complications.
Pain may come from the site of surgical incision, from rib damage or rib removal, from intercostal nerves injury, from incision of pulmonary parenchyma or pleura and from the presence and subsequent irritation due to drainage tubes. Inrercostal nerves mediate nociceptive pain after thoracic surgery from structures of chest wall and the pleura, the phrenic nerve from the diaphragmatic pleura and the vagal nerve from the lung, mediastinum and the mediastinal pleura (1,2). It is believed that the shoulder pain accompanying frequently thoracic procedures is produced by afferent impulses conducted with phrenic nerves. This kind of pain can appear even when thoracic epidural or suprascapular blockade is established (3), but this is not the case if phrenic blockade is performed (4). Sympathetic nerves mediate pain from the pleura to the central nervous system causing visceral pain (1,2). The most common case of persistent pain after thoracotomy is due to damage of myofascial structures (muscle, bone, tendons and ligaments) (5,6). The presence of drainage tubes and residual pleural blood may compress and irritate the intercostal nerves, causing further inflammation and pain. In addition, intercostal nerves may be damaged if sutures or wires are passed around the ribs close to the neurovascular bundle. The subsequent neuralgia is a burning and lancinating pain, which is worsened in the night and by stretching the affected nerve (5). Generally, any movement that causes tension on the incision can increase the severity of pain. Such movements are deep breathing, coughing and extensive body movements.
The usually performed surgical procedures are the classical thoracotomy, the mini-thoracotomy and the video assisted thoracoscopic surgery (VATS). The latter involves the use of trocars. The pain produced from classical thoracotomy procedure is more severe than the pain after thoracoscopic surgery, as indicated from the increased visual analogue scale pain intensity (VASpi) at rest and the increased morphine consumption in the first group (thoracotomy patients) (7). After VATS the postoperative hospital stay is significant shorter. However, VATS is not associated with significant reduction of postoperative chronic pain syndrome, which is probably due to intercostal nerve and chest wall muscle trauma from trocar insertion (8).
The pain severity depends on the location and the length of the thoracotomy incision. Antero-lateral thoracotomy and median sternotomy are associated with less postoperative pain in comparison with the postero-lateral thoracotomy (9). However, the major disadvantage of median sternotomy is the limited access to some intrathoracic structures (10,11). The classical thoracotomy is more painful than the mini thoracotomy, because classical thoracotomy is a longer incision and the tissue trauma is more extended. Muscle sparing incisions have been described, but the surgical view is limited.
If the patient is already on chronic opioid therapy, tolerance is developed and the quality of postoperative pain management with opioids is reduced. Young patients are more vulnerable to postoperative pain, while the elderly patients are more sensitive to systemic opioids. Because the psychological factor is a major component in the process of pain generation, perception and sufferance, good preoperative communication and anxiety decrease can favorably affect this pain dimensions.
Pain management
There are several approaches for the pain management after thoracic surgery. Systemic analgesics can be used alone or in combination between them or in combination with regional techniques. The systemic analgesics commonly used are paracetamol, morphine, NSAID, ketamine or the coadministration of opioids and NSAID. The drug is administrated usually intravenous, but other routes such intramuscular or subcutaneous are acceptable, although not commonly used.
The regional technique usually used is intercostal blockade intraoperatively before the closure of the thoracotomy, or directly postoperatively. The preferred intraoperative injection under direct vision decreases the frequency of complications. Other regional alternatives are epidural blockage (gold standard in major open thoracotomy), the intrathecal or epidural administration of morphine and the paravertebral block.
Systemic approach
Paracetamol—NSAIDs
Paracetamol is classified as mild analgesic and not as NSAID because its anti-inflammatory activity is weak. It is found that paracetamol in patients with thoracic epidural analgesia can decrease post-thoracotomy ipsilateral shoulder pain when given preemptively and regularly during the first 48 hours postoperatively (12).
The pain relief from NSAIDs is due to the inhibition of cyclooxygenase, an enzyme that is involved in the production of prostaglandins, prostacyclins and thromboxans, which are all involved in the generation of pain (13).
The NSAIDs usually used for postoperative pain management are diclofenac, ketorolac, lysine acetyl salicylate, indomethacin, piroxicam, and tenoxicam. NSAIDs affect adversely the coagulation because they cause platelet dysfunction, making systemic bleeding more possible. This effect is independent from the route of administration. Among the others potential adverse effects, the renal dysfunction and the gastrointestinal bleeding are the most important (14-19). The patients with pre-existing renal disease, hypovolemia or treatment with loop-diuretics are more vulnerable to acute renal failure.
Intramuscular diclofenac 75 mg/12 h (20), rectally indomethacin 200 mg/24 h (21) or continuous intravenous lysine acetyl salicylate (7.2 g/24 h) (22) decrease the required quantities of morphine and the postoperative VAS scores. Indeed, the i.v. lysine acetyl salicylate was comparable with i.v. infusion of morphine (40 mg/24 h).
The development of selective COX-2 inhibitors has reduced COX-1 dependent adverse events, such gastrointestinal bleeding, while offering analgesia and decreased opioids requirements and possibly decreased nausea and vomiting (23). In a prospective randomized double-blind placebo-controlled study with patients undergoing thoracotomy and taking epidural patient controlled analgesia (PCA) (T4-T5) for postoperative analgesia for 48 h, the addition of oral celecoxib improved significantly the patient satisfaction and the postoperative pain scores at rest and during coughing (24). However, selective COX-2 inhibitors have not found widespread use, due to concerns of increased severe myocardial ischaemic events.
The inhibition of inflammation due to NSAIDs may be undesirable in specific procedures such as pleurodesis, where the propagation of inflammation is a part of therapeutic approach.
Opioids
The traditional use of opioids for postoperative analgesia after thoracic surgery includes morphine, pethidine (meperidine), fentanyl or tramadol. The route of administration may be intravenous, intramuscular or subcutaneous. Usually, the use of opioids is almost always supplemental to other alternative analgesic approaches. The administered doses depends on the choice of opioid, the route of administration and the possible other supplemental analgesic treatment (Table 1).

Full table
The action of tramadol is mediated through µ-opioid and nonopioid activity. The latter involves increased α2-agonist and serotonergic activity (25). The usual dosage in adult patients postoperatively is 100 mg i.v. as a loading dose, followed by a repetition dosage of 50-100 mg i.v. every 4-6 h.
The major disadvantage of opioids used for postoperative pain treatment is the narrow therapeutic window, with the consequence of nausea, vomiting, somnolence or respiratory depression even with moderate doses. Moreover, patients on a chronic opioid therapy can develop tolerance to these drugs, making the pain relief with their use more difficult. In that case, the use of gabapentin may provide pre-emptive analgesia, limiting the incidence of Chronic Post-Operative Pain Syndrome in these patients. The combination of i.v. opioids and NSAID i.v. has become popular, with satisfactory safety regarding anticoagulation and renal function. In addition there are other regional alternatives that can be used in effective combination with the systemic use of opioids, such as the intercostal, intrapleural, intraspinal and paravertebral blockade with the use of local anesthetics.
Ketamine
Ketamine is a non-competitive antagonist which blocks the ion channel associated with NMDA receptor. By this way the central hyperexcitability of dorsal horn neurons is blocked. The activation of NMDA receptor plays an important role in postinjury central sensitization and hyperalgesia, suggesting that systemic ketamine may be used effectively in treating postoperative pain (26-28).
After thoracic surgery, i.m. administration of ketamine 1 mg/kg resulted in similar pain scores and in weaker respiratory depression in comparison with i.m. pethidine 1 mg/kg (29).
Ketamine is capable of decreasing significantly immediate post-surgical pain after thoracotomy, but has no benefits in preventing chronic pain measured in long-term follow up (post thoracotomy pain syndrome) (30)!
Intravenous administration of ketamine at induction dose 1 mg/kg, followed by infusion at dose 1 mg/kg/h intraoperatively and 1 mg/kg/24 h postoperatively improved immediate postoperative pain, but failed to control the development of chronic pain at 1-2, 6 weeks and 4 months after surgery (30).
The similar results are produced from the addition of epidural ketamine (1.2 mg/h) to preemptive epidural analgesia with levobupivacaine and fentanyl. No difference in the incidence of chronic post-thoracotomy pain at 3 months between the two groups was observed (31).
Regional analgesia
Regional techniques are very important tools in the treatment of postoperative pain after thoracotomy. Intercostal, paravertebral, epidural and spinal route can be used for drug administration. In addition, the local infiltration with ropivacaine 0.75% along the thoracotomy before the incision and after the closure of the surgical trauma can reduce significantly the severity of pain, acting supplemental to the other regional techniques (32).
Intrapleural analgesia has inconsistent efficacy, especially in post thoracotomy patients, who have chest drain tubes. If local anesthetic is injected into the intrapleural space, it will drain from the chest through the drain tube. Blood may also be present in the thoracic cavity, reducing the analgesic effect of intrapleural anesthetics.
Intercostal blockade
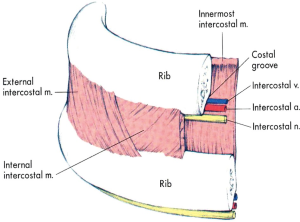
Intercostal blockade interrupts ipsilaterally the transmission of neural impulses from the intercostal sensory and motor fibers of intercostal nerves to and from spinal cord and upper centers. This technique is in practice since many years. By this way, spinal nerves from T1 through T 11 can be blocked effectively decreasing VAS pain scores significantly. Local anesthetics can be infused as a single dose just before closure of the thoracotomy (11,33-36), as a single (37) or multiple (38) percutaneous injections or via an indwelling intercostal catheter (39-41). Intercostal blockade can reduce opioid requirements, however, cannot eliminate them. Supplemental systemic analgesia is almost always needed. Upon removal of pain, the respiratory mechanics improve impressively.
That 3 mL of local anesthetic can easily spread along the groove of intercostal nerve distally and proximally resulting in the nerve block. The most popular site for intercostal nerve blockade in adults is at the angle of the rib, about 6-8 cm from the spinous processes. At the mid-axillary line each nerve gives a lateral cutaneous branch supplying the muscles and skin of the posterolateral thorax and abdomen and an anterior cutaneous branch supplying the muscles and skin of the anterior thorax and abdomen. A block anterior to the mid-axillary line reduces the likelihood of blocking the lateral cutaneous branch (42). If the blockage is performed intraoperatively, the local anesthetic is injected through the thoracic cavity under direct or thoracoscopic vision. For a successful analgesia, blockade at 2 levels above and 2 levels below the dermatomal level of surgical incision is required. If multiple level bilateral blocks are required, then epidural or paravertebral blocks should be more suitable (Figure 1).
Among the main disadvantages of intercostal blocks is the high level of systemic absorption of local anesthetics (39), especially when the blockade is performed at multiple levels. More precisely, the intercostal absorption is the highest in the body. The rich vascular perfusion of the area is responsible for this effect. The local anesthetics that can be used include bupivacaine 0.25-0.5%, lidocaine 1-2% with epinephrine 1/200,000-1/400,000 and ropivacaine 0.5-0.75%. The blockade usually lasts 12±6 h. If epinephrine is added to bupivacaine or ropivacaine, the blockade duration is not prolonged significantly. However, the rate of systemic absorption may be favorably decreased, allowing an increase of dose with the single shot by 30%. With the technique of single shot, the maximum dose of bupivacaine is 2 or 3 mg/kg if epinephrine is added. Maximum lidocaine dose is up to 5 or 7 mg/kg with epinephrine and the maximum ropivacaine single dose is 2.5 or 4 mg/kg with epinephrine. When continuous infusion of local anesthetic is used, the daily dose is greater. Bupivacaine is administered up to 7-10 mg/kg/day, lidocaine up to 20 mg/kg/day and ropivacaine up to 9-12 mg/kg/day.
Significant lower VAS pain scores and lower morphine consumption have been observed after administration of bupivacaine 0.5% 20 mL every 6 h for 24 h via indwelling intercostal catheter. The repeated intercostal bupivacaine administration (400 mg/24 h) resulted in systemic absorption with peak bupivacaine concentration of 1.2 µg/mL, with safe limits of 4 µg/mL (39).
Using neurolytic substances with intercostal blockade, chronic pain conditions such as post-mastectomy pain (T2) and post-thoracotomy pain can be effectively managed.
Paravertebral blockade
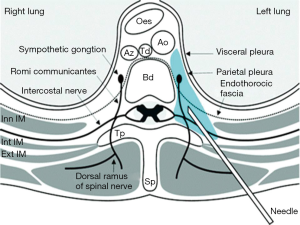
Paravertebral block can cause sympathetic and somatic blockade ispsilaterally via injecting local anesthetics in the vicinity of thoracic spinal nerves which are emerging from the intervertebral foramen. The resultant anesthesia looks like unilateral epidural anesthesia. Paravertebral blockade is more suitable than epidural catheters when there are concerns about the coagulation status.
Choosing the range of thoracic levels, the location of the resulting dermatomal distribution of anesthesia or analgesia can be adjusted to the postoperative pain needs. The injection is performed 2.5 cm laterally from the midline, which is determined by the spinous processes. The target goal is the insertion of needle about 1 cm past the transverse process (Figure 2).
The injected volume of local anesthetic is 3-5 mL and the duration of analgesic effects depends on the choice of local anesthetic. 0.5% ropivacaine establishes its analgesic effects in 15-25 min and lasts for 8-12 h. Increasing the concentration of ropivacaine to 0.75%, the onset time is 10-15 min and the duration of effect 12-18 h. Similarly, when 0.5% bupivacaine (plus epinephrine) is injected, the onset time is 15-25 min with duration of 12-18 h.
Paravertebral blocks can be performed either percutaneously or intraoperatively under direct visual control to avoid complications.
Epidural blockade
Thoracic epidural anesthesia is frequently used for postoperative pain control after thoracotomy or after VATS. It is accepted that thoracic epidural analgesia can reduce significantly the incidence of acute and chronic postoperative pain. However, there is a controversy relating the timing that the epidural analgesia is performed. There are trials that support the preoperative initiation of epidural analgesia, because it causes a significant reduction of the severity and incidence of the postoperative pain syndrome (43,44). On the other side, one trial supports the absence of significance regarding the timing of epidural initiation (45). Epidural blockade cannot block the pain stimuli travelled through the phrenic nerves, resulting in shoulder pain after thoracotomy, although a successful epidural blockade is in place.
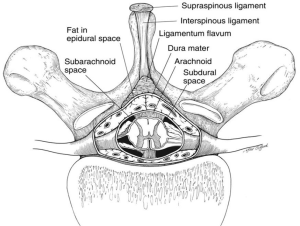
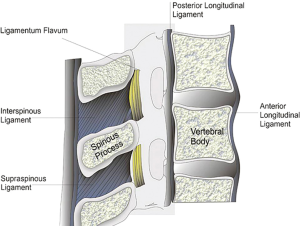
Epidural blockade can be performed through lumbar or thoracic approach. If the entry level is the midthoracic spine (especially T3 to T7), the epidural placement is technically more feasible through the paramedian approach, because the angulation of the spinous processes is more oblique and the openings into the epidural space are much larger (Figures 3,4).
For the achievement of epidural control of postoperative pain several drugs can be used. Local anesthetics can be used alone or in combinations with opioids. The most frequently used local anesthetics are bupivacaine and ropivacaine. Relative dilute solutions are administered for analgesia purposes, while more concentrate solutions are employed for epidural anesthesia. If epinephrine is added to solutions, the durations of action is prolonged. However, the incidence of hypotension is increased, because of beta action of epinephrine causing peripheral vasodilation.
Bupivacaine 0.25% epidurally administered has an onset time of analgesia of 15-20 min and lasts for 160-220 min. Analgesia with ropivacaine 0.5% appears after the same onset time (15-20 min) and lasts 140-180 min.
Bupivacaine can cause excessive cardiotoxicity (hypotension, atrioventricular block, ventricular fibrillation) in the case of intravascular injection, which is refractory to usual resuscitation methods. The cardiotoxicity is due to the strong protein binding of bupivacaine to sodium channels in cardiac cells. Levobupivacaine is the S-enantiomer of bupivacaine, which has the same anesthetic profile with bupivacaine but fortunately lacks its cardiotoxicity.
Local anesthetics can be coadministered with other classes of drugs, aiming the improved quality of neuraxial blockade. Among these agents are clonidine, neostigmine, a variety of opioids, ketamine, tramadol, droperidol, and a new formulation of morphine.
Clonidine can prolong neuraxial blockade and decreases the dose requirements of epidural local anesthetics (46,47), without increasing the hypotension secondary of epidural anesthesia. Moreover, the addition of clonidine can preserve the preoperative lung function in thoracotomy patients (48). The combination of clonidine with opioids acts synergistically, decreasing the dose requirements and the possibility of adverse effects of opioids.
The more recent addition to the list of epidural additives is neostigmine, which is a cholinesterase inhibitor. The inhibition of acetylcholine enzymatic deconstruction and the indirect stimulation of muscarinic and nicotinic receptors into the spinal cord can produce analgesia. Epidurally administered neostigmine can provide postoperative pain relief without respiratory depression, motor impairment or hypotension (49).
Morphine is one of the oldest opioids used epidurally. It can provide pain relief for less than 24 h, requiring alternative analgesic methods or a dose repetition. A new morphine formulation, named Depodur, has the feature of the extended drug release in the epidural space, using a drug-release delivery system called Depofoam. The latter is constituted of microscopic lipid-based particles with internal vesicles, containing the active drug, which is slowly released. This system can provide effective pain relief with minimal adverse effects for up to 48 h (50-52).
Spinal blockade
Intrathecal opioids have been used to supplement the postthoracotomy analgesia. The intrathecal injection of morphine is simple, reliable and has potentially fewer adverse effects in comparison with systemic opioid absorption. Moreover, intrathecal opioids can produce segmental analgesia, resulting in localization of nociception without motor, sensory or autonomic side effects.
Fentanyl is the most frequently used intrathecal lipophilic opioid. When fentanyl is administered intrathecally in single doses of 10-30 µg, the onset of analgesia is rapid (10-20 min) and the action duration is 4-6 h. The cephalad spread is minimal, resulting in small likelihood for respiratory depression (53).
Morphine is a hydrophilic opioid, resulting in slow diffusion into the epidural space and in a high volume of distribution within the spinal cord, with subsequent sustained high concentration in CSF. Low dose intrathecal morphine may cause early or late respiratory depression (up to 24 h). The onset time of analgesia is slow (30-60 min), the duration of action is dose related (13-33 h) and the onset of respiratory depression may be late (it occurs anytime between 3.5 and 12 h after injection with a peak time 6 h after injection). Usually injected single doses of morphine are 0.1-0.2 mg (54,55).
Except respiratory depression, other adverse effects due to intrathecal opioids injections are nausea, pruritus and urinary retention (56-58). Rare cases of spinal cord trauma or nerve trauma, hematoma, infection or inflammatory reaction are associated with the introduction of catheter or needle.
Patient controlled analgesia (PCA) (59)
PCA can approach the near optimal state of analgesia, maintained with minimum sedation and side effects. The patient adjusts the repetition of dose to the analgesic needs, outreaching the minimum effective analgesic concentration. Toxic drug concentrations cannot be reached because the subsequent sedation acts prophylactically by stopping the dose repetition from the patient.
PCA can be used for drug delivery via intravenous (most frequently) or epidural route. PCA is not a good analgesic alternative if the patient is confused and not capable of using the PCA pump handset. Before the initiation of PCA use, a sufficient analgesic state should be established.
In the case of epidural PCA, a solution of L-bupivacaine 0.125 with fentanyl 4 mcg/mL gives satisfactory analgesia. The bolus dose should be 3-5 mL, the lockout period 10-15 min with no background continuous infusion. If the latter is the case, then the bolus dose should be decreased and the lockout period increased!
If PCA is used for intravenous drug administration, it is commonly combined with paravertebral or intercostal nerve blocks. Otherwise, the systemic opioids side effects may limit the dosage, resulting in suboptimal analgesia with subsequent respiratory complications in thoracotomy patients. The bolus doses could be morphine 1-2 mg, fentanyl 10-20 mg, pethidine 10 mg or tramadol 10 mg. The lockout time should not be less than 5-8 min according to the above doses. The background infusion may increase the incidence of respiratory depression and is useful only in opioid tolerant patients.
The major concern with the function of PCA is the respiratory depression. The risk is increased if there is a background infusion, in elderly patients, if concomitant sedatives are administered, in respiratory disease, in obstructive sleep apnea, and if there are operator or equipment errors. The administration of bolus naloxone 400 mg i.v. or more reverses the respiratory depression, and perhaps continuous naloxone infusion may be required, due to its shorter half life.
Chronic postoperative pain syndrome
Some patients develop chronic postoperative pain of significant duration of months or even of years. The incidence of the syndrome is decreasing with the time, however, it is significant high. It can be as high as 80% at 3 months, 75% at 6 months, 61% at 1 year, 36% at 4-5 years and 21% at 6-7 years. Factors affecting the incidence of the syndrome are the age, the gender, the incision type and the high consumption of analgesics during the first post-operative week (60).
This syndrome has a significant neuropathic component and opioid therapy may be ineffective. In contrast, gabapentinoids seem to be effective for relief of chronic pain. Other agents that can be used for the treatment of chronic post-thoracotomy pain are pregabalin, tricyclics antidepressants, serotonine-norepinephrine reputable inhibitors and lidocaine patches (61). Tramadol failed to prevent the development of chronic pain when the patients were followed up in a long term basis (24).
In case of fail of the medical therapy, intercostal nerve blocks and pulsed radiofrequency of the dorsal root ganglion may be useful (62-79). Epidural blockade can alleviate some types of thoracic pain, but there is doubt about its effectiveness in prevention of chronic postoperative pain syndrome (80-94). One effective alternative is spinal cord and peripheral nerve stimulation. The leads are placed for a trial period of several days aiming the proof of significant pain relief (91,95-107). When this proof is given, then the more permanent leads and the externally programmable pulse generator are placed. If the pain relief becomes inadequate, the leads and the generator can be removed.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Conacher ID. Pain relief after thoracotomy. Br J Anaesth 1990;65:806-12. [PubMed]
- Cervero F, Laird JM. Visceral pain. Lancet 1999;353:2145-8. [PubMed]
- Tan N, Agnew NM, Scawn ND, et al. Suprascapular nerve block for ipsilateral shoulder pain after thoracotomy with thoracic epidural analgesia: a double-blind comparison of 0.5% bupivacaine and 0.9% saline. Anesth Analg 2002;94:199-202. [PubMed]
- Scawn ND, Pennefather SH, Soorae A, et al. Ipsilateral shoulder pain after thoracotomy with epidural analgesia: the influence of phrenic nerve infiltration with lidocaine. Anesth Analg 2001;93:260-4. [PubMed]
- Rai PP, Brannon JE. Analgesic considerations for the median sternotomy. In: Gravlee GP, Rauck RL. eds. Pain management in cardiothoracic Surgery. Philadelphia: JB Lippincott Company, 1993:101-24.
- Wallace AM, Wallace MS. Post-mastectomy and postthoracotomy pain. Anesthesiol Clin North Am 1997;15:353-70.
- Kristiina P. Pain After Thoracic Surgery. Helsinki: Yliopistopaino, 2003;11.
- Gottschalk A, Cohen SP, Yang S, et al. Preventing and treating pain after thoracic surgery. Anesthesiology 2006;104:594-600. [PubMed]
- Hazelrigg SR, Landreneau RJ, Boley TM, et al. The effect of muscle-sparing versus standard posterolateral thoracotomy on pulmonary function, muscle strength, and postoperative pain. J Thorac Cardiovasc Surg 1991;101:394-400. [PubMed]
- Baeza OR, Foster ED. Vertical axillary thoracotomy: a functional and cosmetically appealing incision. Ann Thorac Surg 1976;22:287-8. [PubMed]
- de la Rocha AG, Chambers K. Pain amelioration after thoracotomy: a prospective, randomized study. Ann Thorac Surg 1984;37:239-42. [PubMed]
- Mac TB, Girard F, Chouinard P, et al. Acetaminophen decreases early post-thoracotomy ipsilateral shoulder pain in patients with thoracic epidural analgesia: a double-blind placebo-controlled study. J Cardiothorac Vasc Anesth 2005;19:475-8. [PubMed]
- Juan H.. Prostaglandins as modulators of pain. Gen Pharmacol 1978;9:403-9. [PubMed]
- Camu F, Van Lersberghe C, Lauwers MH. Cardiovascular risks and benefits of perioperative nonsteroidal anti-inflammatory drug treatment. Drugs 1992;44 Suppl 5:42-51. [PubMed]
- Kenny GN. Potential renal, haematological and allergic adverse effects associated with nonsteroidal anti-inflammatory drugs. Drugs 1992;44 Suppl 5:31-6. [PubMed]
- Johnson AG, Seidemann P, Day RO. NSAID-related adverse drug interactions with clinical relevance. An update. Int J Clin Pharmacol Ther 1994;32:509-32. [PubMed]
- Bugge JF. Renal effects and complications of NSAIDs for routine post-operative pain relief: increased awareness of a real problem is needed. Baillieres Clinical Anaesthesiology 1995;9:483-92.
- van Wijk CH, Meintjes WA. Subjective Narcosis Assessment Scale: measuring the subjective experience of nitrogen narcosis. Undersea Hyperb Med 2014;41:557-63. [PubMed]
- Lee A, Cooper MG, Craig JC, et al. The effects of nonsteroidal anti-inflammatory drugs (NSAIDs) on postoperative renal function: a meta-analysis. Anaesth Intensive Care 1999;27:574-80. [PubMed]
- Rhodes M, Conacher I, Morritt G, et al. Nonsteroidal antiinflammatory drugs for postthoracotomy pain. A prospective controlled trial after lateral thoracotomy. J Thorac Cardiovasc Surg 1992;103:17-20. [PubMed]
- Pavy T, Medley C, Murphy DF. Effect of indomethacin on pain relief after thoracotomy. Br J Anaesth 1990;65:624-7. [PubMed]
- Jones RM, Cashman JN, Foster JM, et al. Comparison of infusions of morphine and lysine acetyl salicylate for the relief of pain following thoracic surgery. Br J Anaesth 1985;57:259-63. [PubMed]
- McCrory CR, Lindahl SG. Cyclooxygenase inhibition for postoperative analgesia. Anesth Analg 2002;95:169-76. [PubMed]
- Senard M, Deflandre EP, Ledoux D, et al. Effect of celecoxib combined with thoracic epidural analgesia on pain after thoracotomy. Br J Anaesth 2010;105:196-200. [PubMed]
- Scott LJ, Perry CM. Tramadol: a review of its use in perioperative pain. Drugs 2000;60:139-76. [PubMed]
- Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain 1991;44:293-9. [PubMed]
- Chia YY, Liu K, Liu YC, et al. Adding ketamine in a multimodal patient-controlled epidural regimen reduces postoperative pain and analgesic consumption. Anesth Analg 1998;86:1245-9. [PubMed]
- Chow TK, Penberthy AJ, Goodchild CS. Ketamine as an adjunct to morphine in postthoracotomy analgesia: an unintended N-of-1 study. Anesth Analg 1998;87:1372-4. [PubMed]
- Dich-Nielsen JO, Svendsen LB, Berthelsen P. Intramuscular low-dose ketamine versus pethidine for postoperative pain treatment after thoracic surgery. Acta Anaesthesiol Scand 1992;36:583-7. [PubMed]
- Dualé C, Sibaud F, Guastella V, et al. Perioperative ketamine does not prevent chronic pain after thoracotomy. Eur J Pain 2009;13:497-505. [PubMed]
- Ryu HG, Lee CJ, Kim YT, et al. Preemptive low-dose epidural ketamine for preventing chronic postthoracotomy pain: a prospective, double-blinded, randomized, clinical trial. Clin J Pain 2011;27:304-8. [PubMed]
- de Carvalho AL, Castellana FB, Oliva Gatt BE, et al. Preemptive activity of incision infiltration with 0.75% ropivacaine in patients submitted to inguinal hernia repair. Rev dor São Paulo 2011;12:321-6. Available online:
- Delilkan AE, Lee CK, Yong NK, et al. Post-operative local analgesia for thoracotomy with direct bupivacaine intercostal blocks. Anaesthesia 1973;28:561-7. [PubMed]
- Galway JE, Caves PK, Dundee JW. Effect of intercostal nerve blockade during operation on lung function and the relief of pain following thoracotomy. Br J Anaesth 1975;47:730-5. [PubMed]
- Kaplan JA, Miller ED Jr. gallagher EG Jr. Postoperative analgesia for thoracotomy patients. Anesth Analg 1975;54:773-7. [PubMed]
- Toledo-Pereyra LH, DeMeester TR. Prospective randomized evaluation of intrathoracic intercostal nerve block with bupivacaine on postoperative ventilatory function. Ann Thorac Surg 1979;27:203-5. [PubMed]
- Swann DG, Armstrong PJ, Douglas E, et al. The alkalinisation of bupivacaine for intercostal nerve blockade. Anaesthesia 1991;46:174-6. [PubMed]
- Asantila R, Rosenberg PH, Scheinin B. Comparison of different methods of postoperative analgesia after thoracotomy. Acta Anaesthesiol Scand 1986;30:421-5. [PubMed]
- Chan VW, Chung F, Cheng DC, et al. Analgesic and pulmonary effects of continuous intercostal nerve block following thoracotomy. Can J Anaesth 1991;38:733-9. [PubMed]
- Deneuville M, Bisserier A, Regnard JF, et al. Continuous intercostal analgesia with 0.5% bupivacaine after thoracotomy: a randomized study. Ann Thorac Surg 1993;55:381-5. [PubMed]
- Dryden CM, McMenemin I, Duthie DJ. Efficacy of continuous intercostal bupivacaine for pain relief after thoracotomy. Br J Anaesth 1993;70:508-10. [PubMed]
- Techniques in thoracic anaesthesia. Intercostal nerve block. In: Wilkinson JN, Pennefather SH, McCahon RA. eds. Thoracic Anaesthesia. Oxford: Oxford University Press, 2011;700-2.
- Sentürk M, Ozcan PE, Talu GK, et al. The effects of three different analgesia techniques on long-term postthoracotomy pain. Anesth Analg 2002;94:11-5. [PubMed]
- Obata H, Saito S, Fujita N, et al. Epidural block with mepivacaine before surgery reduces long-term post-thoracotomy pain. Can J Anaesth 1999;46:1127-32. [PubMed]
- Ochroch EA, Gottschalk A, Augostides J, et al. Long-term pain and activity during recovery from major thoracotomy using thoracic epidural analgesia. Anesthesiology 2002;97:1234-44. [PubMed]
- Landau R, Schiffer E, Morales M, et al. The dose-sparing effect of clonidine added to ropivacaine for labor epidural analgesia. Anesth Analg 2002;95:728-34. [PubMed]
- Kayacan N, Arici G, Karsli B, et al. Patient-controlled epidural analgesia in labour: the addition of fentanyl or clonidine to bupivacaine. Agri 2004;16:59-66. [PubMed]
- Matot I, Drenger B, Weissman C, et al. Epidural clonidine, bupivacaine and methadone as the sole analgesic agent after thoracotomy for lung resection. Anaesthesia 2004;59:861-6. [PubMed]
- Roelants F, Lavand'homme PM, Mercier-Fuzier V. Epidural administration of neostigmine and clonidine to induce labor analgesia: evaluation of efficacy and local anesthetic-sparing effect. Anesthesiology 2005;102:1205-10. [PubMed]
- Gambling D, Hughes T, Martin G, et al. A comparison of Depodur, a novel, single-dose extended-release epidural morphine, with standard epidural morphine for pain relief after lower abdominal surgery. Anesth Analg 2005;100:1065-74. [PubMed]
- Viscusi ER, Martin G, Hartrick CT, et al. Forty-eight hours of postoperative pain relief after total hip arthroplasty with a novel, extended-release epidural morphine formulation. Anesthesiology 2005;102:1014-22. [PubMed]
- Carvalho B, Riley E, Cohen SE, et al. Single-dose, sustained-release epidural morphine in the management of postoperative pain after elective cesarean delivery: results of a multicenter randomized controlled study. Anesth Analg 2005;100:1150-8. [PubMed]
- Hindle A.. Intrathecal opioids in the management of acute postoperative pain. Contin Educ Anaesth Crit Care Pain 2008;8:81-5.
- Neustein SM, Cohen E. Intrathecal morphine during thoracotomy, Part II: Effect on postoperative meperidine requirements and pulmonary function tests. J Cardiothorac Vasc Anesth 1993;7:157-9. [PubMed]
- Mason N, Gondret R, Junca A, et al. Intrathecal sufentanil and morphine for post-thoracotomy pain relief. Br J Anaesth 2001;86:236-40. [PubMed]
- Bridenbaugh PO, Greene NM, Brull SJ. Spinal (subarachnoidal) neural blockade. In: Cousins MJ, Bridenbaugh PO. eds. Neural Blockade in Clinical Anesthesia and Management of Pain, 3rd ed. Philadelphia: Lippincott-Raven, 1998:203-41.
- Carr DB, Cousins M J. Spinal route of analgesia: opioids and future options. In: Cousins MJ, Bridenbaugh PO. eds. Neural Blockade in Clinical Anesthesia and Management of Pain, 3rd ed. Philadelphia: Lippincott-Raven, 1998:915-83.
- Cousins MJ, Veering BT. Epidural neural blockade. In: Cousins MJ, Bridenbaugh PO. eds. Neural Blockade in Clinical Anesthesia and Management of Pain, 3rd ed. Philadelphia: Lippincott-Raven, 1998;243-322.
- Techniques in thoracic anaesthesia. In: Wilkinson JN, Pennefather SH, McCahon RA. eds. Thoracic Anaesthesia. Oxford: Oxford University Press, 2011:704-6.
- Critical care and the post-operative thoracic patient. Pain management. In: Wilkinson JN, Pennefather SH, McCahon RA. eds. Thoracic Anaesthesia. Oxford: Oxford University Press, 2011:576-8.
- Dworkin RH, O'Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc 2010;85:S3-14. [PubMed]
- Doi K, Nikai T, Sakura S, et al. Intercostal nerve block with 5% tetracaine for chronic pain syndromes. J Clin Anesth 2002;14:39-41. [PubMed]
- Cohen SP, Sireci A, Wu CL, et al. Pulsed radiofrequency of the dorsal root ganglia is superior to pharmacotherapy or pulsed radiofrequency of the intercostal nerves in the treatment of chronic postsurgical thoracic pain. Pain Physician 2006;9:227-35. [PubMed]
- Kioumis IP, Zarogoulidis K, Huang H, et al. Pneumothorax in cystic fibrosis. J Thorac Dis 2014;6:S480-7. [PubMed]
- Kuhajda I, Zarogoulidis K, Kougioumtzi I, et al. Tube thoracostomy; chest tube implantation and follow up. J Thorac Dis 2014;6:S470-9. [PubMed]
- Manika K, Kioumis I, Zarogoulidis K, et al. Pneumothorax in sarcoidosis. J Thorac Dis 2014;6:S466-9. [PubMed]
- Kuhajda I, Zarogoulidis K, Kougioumtzi I, et al. Penetrating trauma. J Thorac Dis 2014;6:S461-5. [PubMed]
- Visouli AN, Zarogoulidis K, Kougioumtzi I, et al. Catamenial pneumothorax. J Thorac Dis 2014;6:S448-60. [PubMed]
- Huang Y, Huang H, Li Q, et al. Transbronchial lung biopsy and pneumothorax. J Thorac Dis 2014;6:S443-7. [PubMed]
- Terzi E, Zarogoulidis K, Kougioumtzi I, et al. Acute respiratory distress syndrome and pneumothorax. J Thorac Dis 2014;6:S435-42. [PubMed]
- Boskovic T, Stojanovic M, Stanic J, et al. Pneumothorax after transbronchial needle biopsy. J Thorac Dis 2014;6:S427-34. [PubMed]
- Li Z, Huang H, Li Q, et al. Pneumothorax: observation. J Thorac Dis 2014;6:S421-6. [PubMed]
- Huang Y, Huang H, Li Q, et al. Approach of the treatment for pneumothorax. J Thorac Dis 2014;6:S416-20. [PubMed]
- Browning RF, Parrish S, Sarkar S, et al. Bronchoscopic interventions for severe COPD. J Thorac Dis 2014;6:S407-15. [PubMed]
- Machairiotis N, Kougioumtzi I, Dryllis G, et al. Laparoscopy induced pneumothorax. J Thorac Dis 2014;6:S404-6. [PubMed]
- Ouellette DR, Parrish S, Browning RF, et al. Unusual causes of pneumothorax. J Thorac Dis 2014;6:S392-403. [PubMed]
- Parrish S, Browning RF, Turner JF Jr, et al. The role for medical thoracoscopy in pneumothorax. J Thorac Dis 2014;6:S383-91. [PubMed]
- Terzi E, Zarogoulidis K, Kougioumtzi I, et al. Human immunodeficiency virus infection and pneumothorax. J Thorac Dis 2014;6:S377-82. [PubMed]
- Zarogoulidis P, Kioumis I, Pitsiou G, et al. Pneumothorax: from definition to diagnosis and treatment. J Thorac Dis 2014;6:S372-6. [PubMed]
- Tsakiridis K, Mpakas A, Kesisis G, et al. Lung inflammatory response syndrome after cardiac-operations and treatment of lornoxicam. J Thorac Dis 2014;6 Suppl 1:S78-98. [PubMed]
- Tsakiridis K, Zarogoulidis P, Vretzkakis G, et al. Effect of lornoxicam in lung inflammatory response syndrome after operations for cardiac surgery with cardiopulmonary bypass. J Thorac Dis 2014;6 Suppl 1:S7-20. [PubMed]
- Argiriou M, Kolokotron SM, Sakellaridis T, et al. Right heart failure post left ventricular assist device implantation. J Thorac Dis 2014;6 Suppl 1:S52-9. [PubMed]
- Madesis A, Tsakiridis K, Zarogoulidis P, et al. Review of mitral valve insufficiency: repair or replacement. J Thorac Dis 2014;6 Suppl 1:S39-51. [PubMed]
- Siminelakis S, Kakourou A, Batistatou A, et al. Thirteen years follow-up of heart myxoma operated patients: what is the appropriate surgical technique? J Thorac Dis 2014;6 Suppl 1:S32-8. [PubMed]
- Foroulis CN, Kleontas A, Karatzopoulos A, et al. Early reoperation performed for the management of complications in patients undergoing general thoracic surgical procedures. J Thorac Dis 2014;6 Suppl 1:S21-31. [PubMed]
- Nikolaos P, Vasilios L, Efstratios K, et al. Therapeutic modalities for Pancoast tumors. J Thorac Dis 2014;6 Suppl 1:S180-93. [PubMed]
- Koutentakis M, Siminelakis S, Korantzopoulos P, et al. Surgical management of cardiac implantable electronic device infections. J Thorac Dis 2014;6 Suppl 1:S173-9. [PubMed]
- Spyratos D, Zarogoulidis P, Porpodis K, et al. Preoperative evaluation for lung cancer resection. J Thorac Dis 2014;6 Suppl 1:S162-6. [PubMed]
- Porpodis K, Zarogoulidis P, Spyratos D, et al. Pneumothorax and asthma. J Thorac Dis 2014;6 Suppl 1:S152-61. [PubMed]
- Panagopoulos N, Leivaditis V, Koletsis E, et al. Pancoast tumors: characteristics and preoperative assessment. J Thorac Dis 2014;6 Suppl 1:S108-15. [PubMed]
- Visouli AN, Darwiche K, Mpakas A, et al. Catamenial pneumothorax: a rare entity? Report of 5 cases and review of the literature. J Thorac Dis 2012;4 Suppl 1:17-31. [PubMed]
- Zarogoulidis P, Chatzaki E, Hohenforst-Schmidt W, et al. Management of malignant pleural effusion by suicide gene therapy in advanced stage lung cancer: a case series and literature review. Cancer Gene Ther 2012;19:593-600. [PubMed]
- Papaioannou M, Pitsiou G, Manika K, et al. COPD assessment test: a simple tool to evaluate disease severity and response to treatment. COPD 2014;11:489-95. [PubMed]
- Boskovic T, Stanic J, Pena-Karan S, et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis 2014;6 Suppl 1:S99-107. [PubMed]
- Papaiwannou A, Zarogoulidis P, Porpodis K, et al. Asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): current literature review. J Thorac Dis 2014;6 Suppl 1:S146-51. [PubMed]
- Zarogoulidis P, Porpodis K, Kioumis I, et al. Experimentation with inhaled bronchodilators and corticosteroids. Int J Pharm 2014;461:411-8. [PubMed]
- Bai C, Huang H, Yao X, et al. Application of flexible bronchoscopy in inhalation lung injury. Diagn Pathol 2013;8:174. [PubMed]
- Zarogoulidis P, Kioumis I, Porpodis K, et al. Clinical experimentation with aerosol antibiotics: current and future methods of administration. Drug Des Devel Ther 2013;7:1115-34. [PubMed]
- Zarogoulidis P, Pataka A, Terzi E, et al. Intensive care unit and lung cancer: when should we intubate? J Thorac Dis 2013;5 Suppl 4:S407-12. [PubMed]
- Hohenforst-Schmidt W, Petermann A, Visouli A, et al. Successful application of extracorporeal membrane oxygenation due to pulmonary hemorrhage secondary to granulomatosis with polyangiitis. Drug Des Devel Ther 2013;7:627-33. [PubMed]
- Zarogoulidis P, Kontakiotis T, Tsakiridis K, et al. Difficult airway and difficult intubation in postintubation tracheal stenosis: a case report and literature review. Ther Clin Risk Manag 2012;8:279-86. [PubMed]
- Zarogoulidis P, Tsakiridis K, Kioumis I, et al. Cardiothoracic diseases: basic treatment. J Thorac Dis 2014;6 Suppl 1:S1. [PubMed]
- Kolettas A, Grosomanidis V, Kolettas V, et al. Influence of apnoeic oxygenation in respiratory and circulatory system under general anaesthesia. J Thorac Dis 2014;6 Suppl 1:S116-45. [PubMed]
- Turner JF, Quan W, Zarogoulidis P, et al. A case of pulmonary infiltrates in a patient with colon carcinoma. Case Rep Oncol 2014;7:39-42. [PubMed]
- Machairiotis N, Stylianaki A, Dryllis G, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol 2013;8:194. [PubMed]
- Tsakiridis K, Zarogoulidis P.. An interview between a pulmonologist and a thoracic surgeon-Pleuroscopy: the reappearance of an old definition. J Thorac Dis 2013;5 Suppl 4:S449-51. [PubMed]
- Huang H, Li C, Zarogoulidis P, et al. Endometriosis of the lung: report of a case and literature review. Eur J Med Res 2013;18:13. [PubMed]