
Vasoplegia following heart transplantation and left ventricular assist device explant is not associated with inferior outcomes
Introduction
Vasoplegia after cardiac surgery describes the syndrome of refractory hypotension, reduced systemic vascular resistance and normal or elevated cardiac output that is seen to develop in some patients, requiring high-dose vasopressors in order to prevent end organ dysfunction (1). Previous studies have reported vasoplegia to occur more often after heart transplantation (HTx) compared to other cardiac surgeries. It is thought this is due to the heightened pro-inflammatory state that exists in advanced heart failure, combined with the inflammatory response that ensues upon reperfusion and release of donor antigen (2).
Several studies have demonstrated durable continuous-flow left ventricular assist device (CF-LVAD) to be an independent predictor for the development of vasoplegia (3-7). These studies have reported that vasoplegia is associated with inferior outcomes following HTx, including decreased survival.
With the evolution of LVAD technology and the improved survival and quality of life observed, there has been an increase in use of durable LVADs as a bridge to transplantation (5). This will result in more patients undergoing HTx with a durable LVAD. It is therefore important to further understand the association between transplantation of patients with a durable LVAD and the development of postoperative vasoplegia.
The aim of this study was to examine the population of patients with a durable CF-LVAD undergoing HTx at a single centre and determine factors associated with the development of vasoplegia, and its impact on patient outcomes.
Methods
Patient population
All patients undergoing orthotopic HTx with a durable CF-LVAD at Royal Papworth Hospital, United Kingdom, were included in this study. There were no patients excluded. We are an adult cardiac centre and do not receive paediatric referrals. The study period extended from 1st January 2013 until 31st December 2018. All patients in this study period undergoing transplantation bridged with an LVAD were implanted with the HeartWare ventricular assist device—HVAD (HeartWare International Inc., Framingham, MA, USA). Patient demographics, peri-operative course and outcome data including incidence of complications, length of hospital and ICU stay, and survival data were retrospectively retrieved from our surgical, transplant and intensive care databases. These databases are prospectively maintained and there was no missing data for these patients. The Royal Papworth Hospital Research Governance Department approved the study, waiving the requirement for patient consent.
Over the period of study, the HVAD implantation was performed either by full sternotomy or through a mini-thoracotomy and partial sternotomy, but typically utilised an anticoagulation-free, off-pump technique (8).
Transplant procedure
All patients with a durable CF-LVAD had a computed tomography scan prior to listing for transplantation in order to allow for surgical planning. Hearts were procured following two separate pathways—donation after brain death (DBD) and donation after circulatory death (DCD).
DCD hearts were transported with machine perfusion on the Transmedics Organ Care System™, permitting a period of assessment and observation, whilst simultaneously reducing the ischaemic time; whilst DBD hearts underwent standard cold storage. The transplant procedure was performed by 2 consultant surgeons using a standard bicaval implantation technique. No patient requires a period of circulatory arrest. Following the operation, transplant recipients were admitted to the intensive care unit and managed in accordance with the transplant unit policy. Regarding immunosuppression, patients receive induction with mycophenolate mofetil, methylprednisolone and three doses of rabbit anti-human thymocyte globulin. Maintenance immunosuppression is with prednisolone, tacrolimus and mycophenolate mofetil. Continuous haemodynamic parameters were obtained with invasive arterial, central venous and Swan Ganz catheters.
Definitions
Patients were divided into two groups on the basis of the diagnosis of vasoplegia. There is currently no consensus definition of vasoplegia, so we used previously published literature as a guide (4,7): vasoplegia was defined on the basis of requiring high dose, two agent vasopressor over the first 24 hours following transplantation to maintain a normal SVR and a mean arterial pressure >70 mmHg (average doses over the first 24 hours of norepinephrine >0.5 μg/kg/min and vasopressin >1 U/h). All patients were determined to have appropriate cardiac function with a cardiac index >2.0 L/min/m2 (calculated using Swan Ganz catheter with thermodilution technique). Consideration was given to exclude other causes of low SVR for example, sepsis.
Inotrope and Vasopressor-Inotrope scores were calculated using the following equations (9):
- Inotrope score = dopamine dose (mcg/kg/min) + dobutamine dose (mcg/kg/min) +100× epinephrine dose (mcg/kg/min);
- Vasopressor-Inotrope score = Inotrope score +10× enoximone dose (mcg/kg/min) +10,000× vasopressin dose (units/kg/min) +100× norepinephrine dose (mcg/kg/min).
For daily scores presented in the results, the total dose of each inotrope/vasopressor administered over the 24-hour period was divided by time to arrive at the score.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 7.03 (GraphPad Software, Inc., San Diego, CA), and R 3.4.4 (R Core Team, Vienna, Austria). The Kaplan-Meier method was used to plot the patient survival rates, with the log-rank (Mantel-Cox) test used to compare groups. Univariate analyses were performed. For comparison of groups, continuous variables were analysed with the Mann-Whitney U test if not normally distributed, summarised with median and interquartile range, and with the Students t-test if normally distributed. Categorical variables were analysed with Fisher’s exact test. P<0.05 was considered statistically significant.
Results
Recipient characteristics
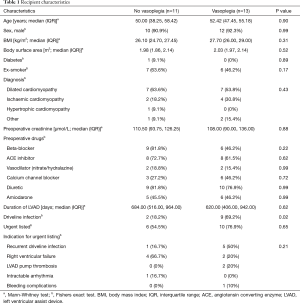
Over the 5-year study period 263 HTx were performed. Of these, 24 patients were supported on HVAD at the time of transplantation. Of these, 13 (54.2%) were determined to have had vasoplegia as per the definition described above. Recipient characteristics are summarised in Table 1. There was no statistically significant difference in the median duration of LVAD support between the two groups (684 vs. 620 days; P=0.62). A greater proportion of patients developing vasoplegia had experienced driveline infections (69.2% vs. 18.2%; P=0.02). Recurrent severe driveline infection was the commonest indication for urgent listing in the patients subsequently developing vasoplegia. No patient had a positive blood culture result over the early post-operative period.
Full table
Donor demographics
Donor demographics are summarised in Table 2. There were no identified differences in the donor characteristics between the two groups.

Full table
Operative and post-reperfusion details
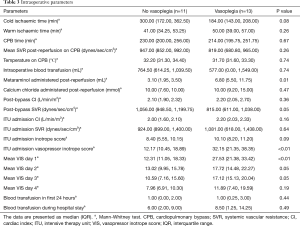
Intraoperative and post-reperfusion details are summarised in Table 3. There were no significant differences in cold and warm ischemic times, median cardiopulmonary bypass (CPB) time or volume of blood transfusion between the groups.
Full table
Post reperfusion of the donor heart, there was a significantly greater amount of metaraminol administered to patients developing vasoplegia (P=0.01). Despite having a similar cardiac index post-CPB (median of 2.10 vs. 2.20 P=0.36), the post-CPB SVR was significantly lower (median 815 vs. 1,056 dynes/sec/cm5; P=0.05). On admission to the intensive care unit (ICU), inotropic and vasopressor support was expectedly greater for patients developing vasoplegia [vasopressor-inotrope score: 32.64 vs. 11.64 (P<0.01)]. The mean vasopressor-inotrope score (VIS) was calculated each day for the first 4 days following transplant and was significantly greater for patients developing vasoplegia until day 4. No patients in this series received adjuncts to treat vasoplegia, such as methylene blue or ascorbic acid.
Patient outcomes
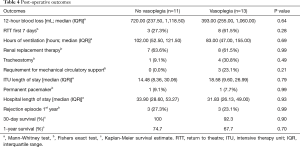
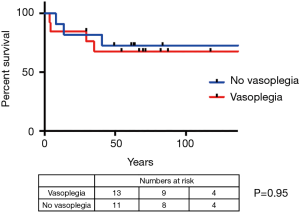
Patient outcomes are summarised in Table 4. Three patients with vasoplegia (23.1%) required mechanical circulatory support (2× right ventricular assist device and 1× veno-arterial extracorporeal membrane oxygenation), whilst no patient in the group without vasoplegia required mechanical circulatory support (P=0.21). There was also no significant difference in survival (Figure 1). The overall 1-year survival for HTx at our centre was 89.3% over this period, during which 263 heart transplants were performed.
Full table
Discussion
In this single centre, retrospective observational study we have examined outcomes following HTx in patients supported preoperatively with a durable CF-LVAD, to assess the impact of vasoplegia on outcomes. We have demonstrated that vasoplegia is common in this patient population with an incidence of 54.2%. It is associated with a history of recurrent driveline infections in the recipient. The impact of vasoplegia, which develops perioperatively, is a requirement for significantly greater vasopressor support persists until the 4th post-operative day. In our small series, 30-day and 1-year survival was not inferior for patients developing vasoplegia, albeit lower than survival for recipients not bridged with an LVAD.
With the disparity between supply and demand for donor organs, use of LVADs as a bridge to transplantation (BTT) has continued to increase. Use of LVADs has been demonstrated to reduce HTx waiting list mortality, while significantly improving patient quality of life, functional status and end organ function (10,11). A recent meta-analysis of clinical outcomes reported estimated actuarial survival after CF-LVAD implantation, regardless of indication, ranging from 56% to 87% at 1 year, 43% to 84% at 2 years, and 47% at 4 years (12).
It has been recognised that patients with an LVAD at the time of transplantation have a significantly greater incidence of vasoplegia postoperatively (4,6,7,13). Vasoplegia is usually present immediately after surgery or within the first 6 hours postoperatively, as we have observed in our series (14). If untreated, this haemodynamic state results in prolonged organ malperfusion and ischaemia, eventually leading to organ dysfunction and failure (15). Treatment typically involves administration of high dose vasoconstrictors, with some advocating use of methylene blue or high dose vitamin B12 in refractory case (14). Being aware of the significant association of vasoplegia in LVAD explant HTx recipients can enable cardiothoracic anaesthetists to be prepared with these treatments to enable rapid and aggressive treatment when it becomes apparent that the patient is becoming vasoplegic—with early signs present following reperfusion of the donor heart. We advocate early floating of a pulmonary artery catheter as soon as surgical anastomoses are finalised, in order to optimise management. Our practice now involves considering administration of methylene blue or high dose vitamin B12 in such patients.
Vasoplegia is reported to occur with an incidence of between 8–25% in the general cardiac surgical population, and is significantly more prevalent following HTx with an incidence as high as 54% reported (1,15). Whilst the precise mechanism for vasoplegia following cardiac surgery has yet to be fully elucidated it is believed to relate to a generalised activation of multiple vasodilatory pathways including production of nitric oxide and activation of inhibitory vascular smooth muscle ion channels, resulting in an inability to maintain vascular tone (5). The increased vasoplegia risk with HTx is thought to relate to an elevation of inflammatory cytokines (tumour necrosis factor-α and interleukin-6) that is observed in advanced heart failure, together with the enhanced systemic inflammatory response that is initiated upon reperfusion and exposure to donor antigens (2,16). Furthermore, there is an association with prolonged cardiopulmonary bypass duration and development of vasoplegia (14). The association between CF-LVAD and vasoplegia is thought to relate to maladaptive structural changes in blood vessels consequent to non-pulsatile flow, that result in a disruption of neuro-hormonal and sympathetic responsiveness of the endothelium and vascular smooth muscle (17). Furthermore, the increased technical complexity of the operation and longer duration are also thought to contribute (18). Our series has suggested that driveline infection may in fact be an important determinant for the development of vasoplegia with the release of bacterial antigens at the time of CF-LVAD explant. One study investigated the impact of induction immunosuppression in the context of driveline infection and found that there was no association with increased morbidity suggesting that this is not likely contributory to the observed effect (19).
The concern regarding vasoplegia in the post-transplant period, is the association with inferior outcomes (5). Several studies have observed increased early morbidity and mortality in patients with vasoplegia following transplantation. Byrne described patients with vasoplegia requiring more post-operative mechanical circulatory support, a higher incidence of re-exploration for bleeding and an increased in-hospital mortality (25% vs. 9%) (4). Patarroyo observed significantly higher incidence of re-exploration for bleeding and mediastinitis in patients with vasoplegia, and this was associated with a prolonged ICU and hospital length of stay and a significantly elevated 30-day mortality (17.1% vs. 3.2% P<0.01) (6). Similar findings were reported by Truby who observed a significantly elevated 30-day mortality in patients with vasoplegia (13.64% vs. 0.84% P<0.01) (3). We observed a 30-day mortality of 7.7% in our patients with vasoplegia. Chan aimed to examine longer term outcomes and observed similar 1-year survival for patients who had vasoplegia (88.6% vs. 92.0% P=0.34) although the authors reported a 19.6% loss to follow-up (7). These results suggest that the impact of vasoplegia is focused in the early post-operative period and if patients can be supported through the physiological insult, there is no longer term impact on survival.
The International Society of Heart and Lung Transplant (ISHLT) registry analysis suggests that there is a non-significant reduction in survival for patients undergoing HTx with a durable LVAD (20). However, it is also recognised that patients transplanted with LVAD-complications have inferior survival (21). It is important to consider differences between practice in the UK and internationally. In the UK, patients with a durable LVAD remain a low priority for transplantation unless there are complications when they are placed on the urgent waiting list. Hence why only 34.8% of patients with a durable LVAD were transplanted electively. As a result, patients are typically supported on durable LVADs for long periods. It has been described that there is inferior survival with longer duration of LVAD support. John et al. for example, observed a 1-year survival of 84% for patients supported for <180 days compared with 79% for patients supported for >1-year (22). It should be noted that in that study the median duration of LVAD support was only 151 days which is significantly lower than that in our series—with a median support of 682 days (range, 107–1,414 days). It may therefore be that inferior survival for patients transplanted with a durable LVAD is a confounding factor that has not been fully taken into consideration by other studies reporting inferior outcomes with patients developing vasoplegia postoperatively.
In our series patients undergoing transplantation supported on HVAD had a 54.2% incidence of vasoplegia. We have observed driveline infection to be associated with the development of vasoplegia in our HVAD population. Our relatively high incidence of driveline infection, we attribute to the long period of support these patients received on LVAD prior to transplantation. Our ability to detect other important predictors may be limited by the number of patients in our series. Previous studies have observed association with the development of vasoplegia and: elevated BMI, higher body surface area, longer ischaemic times, longer cardiopulmonary bypass duration and blood product transfusion (3,4,6,7). These studies have included high proportions of patients not supported by durable LVAD and these factors may be more important in the non-LVAD recipients. Papworth hospital has the world’s leading experience on HTx following DCD, having recently performed our 70th transplant (23). Nine of our recipients in this series received a DCD heart, and there was no association with an increased incidence of vasoplegia, although this conclusion is limited by the small number of patients included to date.
Limitations
This is a retrospective, single centre study with a relatively small number of patients, which potentially limits generalisability of the findings and the ability for us to detect differences. The number of patients means that there may be insufficient power to detect differences between these patient groups which must be acknowledged, and as a result our study offers some insight into this area, but larger numbers would be required to confirm this association. The lack of a consensus definition for vasoplegia somewhat limits direct comparison between studies, however, we have used a definition based on similar published series.
Conclusions
Over half of the patients supported on durable CF-LVAD developed vasoplegia in the immediate post-transplant period in our series—which was associated with the presence of driveline infections. Being aware of this association should increase the vigilance of the medical team and facilitate early aggressive management. The development of vasoplegia appeared not to be associated with inferior 30 day or 1-year survival, suggesting that there are no long-term sequelae of developing this syndrome although we recognise the small number of patients in this study may limit our ability to identify differences. Initial data suggests that DCD does not influence the development of vasoplegia in this patient population. We also observed an inferior survival in patients undergoing HTx bridged with a durable LVAD.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.53). JMA serves as an unpaid editorial board member of Journal of Thoracic Disease from Oct 2019 to Sep 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Royal Papworth Hospital ethical review board approved the study (No. RPH-01324) and waived requirement for patient consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fischer GW, Levin MA. Vasoplegia during cardiac surgery: current concepts and management. Semin Thorac Cardiovasc Surg 2010;22:140-4. [Crossref] [PubMed]
- Wan S, Marchant A, DeSmet JM, et al. Human cytokine responses to cardiac transplantation and coronary artery bypass grafting. J Thorac Cardiovasc Surg 1996;111:469-77. [Crossref] [PubMed]
- Truby LK, Takeda K, Farr M, et al. Incidence and Impact of On-Cardiopulmonary Bypass Vasoplegia During Heart Transplantation. ASAIO J 2018;64:43-51. [Crossref] [PubMed]
- Byrne JG. Risk factors and outcomes for “vasoplegia syndrome” following cardiac transplantation. Eur J Cardiothorac Surg 2004;25:327-32. [Crossref] [PubMed]
- Dardas TF. Impact of Mechanical Circulatory Support on Posttransplant Outcomes. Cardiol Clin 2018;36:551-60. [Crossref] [PubMed]
- Patarroyo M, Simbaqueba C, Shrestha K, et al. Pre-operative risk factors and clinical outcomes associated with vasoplegia in recipients of orthotopic heart transplantation in the contemporary era. J Heart Lung transplant 2012;31:282-7. [Crossref] [PubMed]
- Chan JL, Kobashigawa JA, Aintablian TL, et al. Vasoplegia after heart transplantation: outcomes at 1 year. Interact CardioVasc Thorac Surg 2017;25:212-7. [Crossref] [PubMed]
- Ali J, Balasubramanian S, Berman M, et al. Anticoagulant-Free Off-Pump Left Ventricular Assist Device Implant. Ann Thorac Surg 2018;105:e37-9. [Crossref] [PubMed]
- Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med 2010;11:234-8. [Crossref] [PubMed]
- Jeevanandam V. Are we ready to implant left ventricular assist devices in “less sick” patients? Semin Thorac Cardiovasc Surg 2012;24:8-10. [Crossref] [PubMed]
- Starling RC, Naka Y, Boyle AJ, et al. Results of the post-U.S. Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol 2011;57:1890-8. [Crossref] [PubMed]
- McIlvennan CK, Magid KH, Ambardekar AV, et al. Clinical outcomes after continuous-flow left ventricular assist device: a systematic review. Circ Heart Fail 2014;7:1003-13. [Crossref] [PubMed]
- Sacks LD, Hollander SA, Zhang Y, et al. Vasoplegia after pediatric cardiac transplantation in patients supported with a continuous flow ventricular assist device. J Thorac Cardiovasc Surg 2019;157:2433-40. [Crossref] [PubMed]
- Cakir H, Yurekli I, Celik E, et al. Methylene Blue for Vasoplegic Syndrome After Cardiac Operations. Ann Thorac Surg 2019;107:685-6. [Crossref] [PubMed]
- Chemmalakuzhy J, Costanzo MR, Meyer P, et al. Hypotension, acidosis, and vasodilatation syndrome post-heart transplant: prognostic variables and outcomes. J Heart Lung Transplant 2001;20:1075-83. [Crossref] [PubMed]
- Kofidis T, Strüber M, Wilhelmi M, et al. Reversal of severe vasoplegia with single-dose methylene blue after heart transplantation. J Thorac Cardiovasc Surg 2001;122:823-4. [Crossref] [PubMed]
- Ambardekar AV, Hunter KS, Babu AN, et al. Changes in Aortic Wall Structure, Composition, and Stiffness With Continuous-Flow Left Ventricular Assist Devices: A Pilot Study. Circ Heart Fail 2015;8:944-52. [Crossref] [PubMed]
- Grosman-Rimon L, Billia F, Fuks A, et al. New therapy, new challenges: The effects of long-term continuous flow left ventricular assist device on inflammation. Int J Cardiol 2016;215:424-30. [Crossref] [PubMed]
- Bhatia N, Voelkel AJ, Hussain Z, et al. Safety and Feasibility of Induction Immunosuppression When Driveline Infection Is an Indication for Cardiac Transplantation. Thorac Cardiovasc Surg 2015;63:675-83. [Crossref] [PubMed]
- Lund LH, Khush KK, Cherikh WS, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Heart Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant 2017;36:1037-46. [Crossref] [PubMed]
- Quader MA, Wolfe LG, Kasirajan V. Heart transplantation outcomes in patients with continuous-flow left ventricular assist device-related complications. J Heart Lung Transplant 2015;34:75-81. [Crossref] [PubMed]
- John R, Pagani FD, Naka Y, et al. Post-cardiac transplant survival after support with a continuous-flow left ventricular assist device: impact of duration of left ventricular assist device support and other variables. J Thorac Cardiovasc Surg 2010;140:174-81. [Crossref] [PubMed]
- Page A, Messer S, Large SR. Heart transplantation from donation after circulatory determined death. Ann Cardiothorac Surg 2018;7:75-81. [Crossref] [PubMed]


