
Preoperative dilated esophagus is associated with a high risk of intrathoracic anastomotic leakage for patients with esophageal cancer
Introduction
Esophagus cancer is the sixth most common cause of cancer deaths worldwide (1). Esophagectomy following by lymphadenectomy, is the best choice for resectable disease (2,3). However, it is a highly invasive surgical procedure. Even though the improvements in surgical techniques and perioperative management, the postoperative anastomotic leakage rate remains high (4,5). Several factors have been suggested to be the risk factors for anastomotic leakage (6-8). However, the preoperative condition of the esophagus itself has been ignored or seldom studied. Although an enhanced computed tomography (CT) scan could help us evaluate the clinical T and N stage, the value of barium esophagography in the evaluation of the morphology changes of the esophagus is irreplaceable (9-11). We observed a dominant dilation of the esophagus above the tumor on the esophagography in some esophageal cancer patients in daily clinical work. We previously reported that preoperative cancerous stenosis correlated to poor prognosis in esophagus cancer patients (12). The longer or more severe the stenosis was, the more stimulation from the food the esophagus above the tumor would suffer. The occupation of the food may finally result in the dilation of the esophageal lumen.
In our hospital, the 25 mm circular stapler is the most commonly used device in the intrathoracic gastroesophageal anastomosis. However, in colorectal cancer, the 28 mm and 29 mm circular stapler is the most widely used device (13). More tissues would be bunched up when the circular stapler is too small. The blood supply of the bunched tissues is poor, and it dooms to impair the healing of the anastomosis. Generally, the lower digestive tract has a larger lumen than the esophagus, so it adopted a lager circular stapler to fit its larger lumen. We think this should draw special attention in the patients with a dilated esophagus. The bunching-up effect when applying the circular stapler could increase the risk of anastomotic leakage in patients with a dilated esophagus. On the basis of this hypothesis we proposed, we conducted this retrospective study to find out if the patient with a dilated esophagus suffered from anastomotic leakage more easily when applying the 25 mm circular stapler to make the anastomosis.
Methods
A retrospective study of clinical records of patients who underwent esophagectomy following by intrathoracic gastroesophageal anastomosis from six esophagus surgery medical groups in West China Hospital between January 2014 and December 2017 was performed. The study was approved by the institutional review board of West China Hospital, Sichuan University (No. 20180321). The inclusion criteria were: (I) patients suffered from esophageal squamous cell carcinoma; (II) the anastomosis was performed within the thoracic cavity using 25 mm circular stapler; (III) patients underwent barium esophagography within one month before surgery. The exclusion criteria were: (I) tumors originated from cardia; (II) patients with a positive resection margin after surgery. Patients with postoperative endoscopy or barium esophagography confirmed anastomotic region leakage was assigned to leakage group (LG), while those without the supporting evidence were assigned to no leakage group (NLG).
Measurement of intraluminal diameter
The barium esophagography was done according to the following procedures. Firstly, the patient stood in an upright position, and the barium sulfate was prepared for drinking. Then, the images were collected synchronously when the patient was drinking the barium sulfate. Forty-six or more anteroposterior pictures were collected for every patient. No antispasmodic agent was used during the process. The measurement of the intraluminal diameter of the esophagus was shown in Figure 1. The intrathoracic anastomosis was done about 5 cm above the upper margin of the tumor averagely in our hospital. So we chose this level to carried out the measurement to get as close as possible to the anastomotic site. And if this level were just behind the aortic arch, which may compress the esophagus, the measurement level would change to the upper margin of the aortic arch (Figure 1B). Actually, when the level of 5 cm away from the upper margin of the tumor was just behind the aortic arch, we prefer performing the anastomosis above the aortic arch. It avoids the danger and difficulty of performing the anastomosis behind the aortic arch. So, in these patients, the upper margin of the aortic arch is the place close to the anastomosis rather than the level of 5.0 cm away from the upper margin of the tumor. When the esophageal lumen at the measurement level was filling with the barium sulfate, we got the filling phase of the esophagus. After the passing through of the barium sulfate, we got the mucosal phase of the esophagus. The intraluminal diameter was measured both in the filling and mucosal phase. And all the measurements were accomplished on anteroposterior barium esophagography with the patient in an upright position. All the measurements were performed by one author (Zhuo) independently, and he was blind to the grouping of the patients.
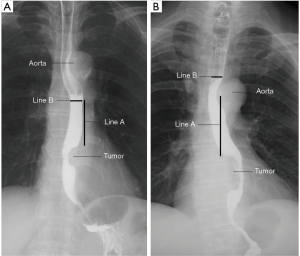
Baseline data collection
The preoperative data included age, gender, body mass index (BMI), main comorbidities such as diabetes and hypertension, patients’ history of smoking and alcohol consumption, duration of dysphagia, and neoadjuvant therapy. Postoperative data included surgeons, surgery type [minimal invasive esophagectomy (MIE) or open esophagectomy (OE)], maximum tumor size, anastomosis position, and pathologic information. Patients were staged according to the eighth edition [2017] of the American Joint Committee on Cancer (AJCC) staging criteria (14).
Statistical analysis
The statistical analyses were performed using SPSS 22.0 (IBM, Armonk, NY, USA). A Student’s t-test was applied to compare the continuously distributed data. Chi-squared test or Fisher’s exact test was applied to dichotomous data, while Mann-Whitney non-parametric U-test was used in the comparison of multi-classification data. The receiver operating characteristic (ROC) curve was used to confirm the cutoff value. A multivariate logistic regression analysis was conducted to identify the factors significantly correlated to the anastomotic leakage. Spearman’s correlation test was used in the detection of the correlation between two variables. P values of less than 0.05 were considered statistically significant.
Results
Over the past four years reviewed, there were 961 patients undergoing esophagectomy following by intrathoracic anastomosis from the six medical groups. Among them, 851 patients underwent esophagectomy for squamous cell cancer of the esophagus. Then, 167 patients were excluded for hand-sewn anastomosis and another 249 patients for no barium esophagography before surgery. Three patients with a positive surgical margin were also excluded. Finally, 432 patients were enrolled in the study. All the anastomoses were accomplished using a 25 mm circular stapler. Thirty-one patients (7.2%) with endoscopy or barium esophagography confirmed anastomotic region leakage was assigned to LG. And the left 401 patients were enrolled in the NLG.
Baseline characteristics
The baseline characteristics of patients enrolled in the study was shown in Table 1. No statistically significant difference was detected between LG and NLG in gender (P=0.962), BMI (P=0.255), smoking history (P=0.718), alcohol consumption history (P=0.703), duration of dysphagia (P=0.528), neoadjuvant therapy (P=0.312), surgery type (P=0.321), maximum size of the tumor (P=0.377), anastomosis position (P=0.986), pathologic T stage (P=0.291), pathologic N stage (P=0.632) and pathologic TNM stage (P=0.576). The surgeon A and B performed over one hundred surgeries, while the left 128 surgeries were performed by the other four surgeons. The incidence of anastomotic leakage was comparable among the six surgeons (P=0.967). The LG seemed to include elder patients, but it didn’t show a statistic difference (P=0.082). More patients in LG had diabetes (12.9% vs. 4.5%), but it also didn’t reach statistic difference (P=0.064). However, LG had a significantly higher prevalence of hypertension (54.8% vs. 18.0%, P<0.001). Although in both the filling phase and the mucosal phase, LG had a greater mean intraluminal diameter than NLG, the difference only reached statistically significant in mucosal phase (P=0.01).
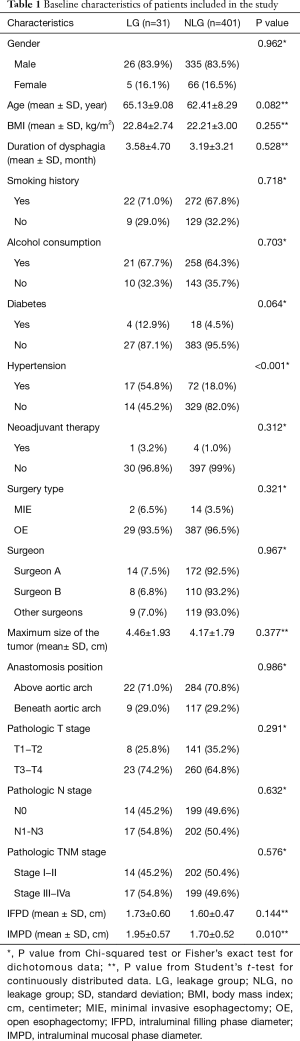
Full table
Intraluminal mucosal phase diameter (IMPD)
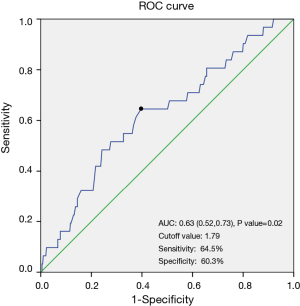
As the ROC curve (Figure 2) showed, the optimal cutoff value for IMPD was 1.79 cm, with an area under the curve (AUC) of 0.63. Its sensitivity was 64.5%, and the specificity was 60.3%. The anastomotic leakage rate was 4.3% in patients with an IMPD of less than 1.79 cm, while 11.2% in patients with an IMPD greater than or equal to 1.79 cm. And the difference reached statistically significant (P=0.007, P value from univariate logistic regression analysis).
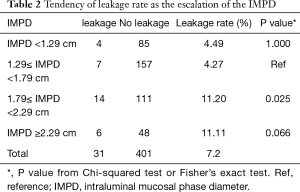
Then, we compared the leakage rate in subgroups with escalating IMPD. We found that as the escalation of the IMPD, the leakage rate also tends to increased (Table 2). The 1.79 cm as the cutoff value was quite an important turning point beyond which the leakage rate would almost triple (from 4.27% to 11.20%). What’s more, the leakage rate remained high in patients with an IMPD over 2.29 cm (11.11%). The dominant difference among the four subgroups in the leakage rate supported the patient with a dilated IMPD was more likely to suffer from the anastomotic leakage.
Full table
Multivariate logistic regression analysis of potential risk factors for anastomotic leakage
To further prove the dilated IMPD was an independent risk factor for the development of anastomotic leakage, a multivariate logistic regression analysis was performed. Factors with a P value of less than 0.1 in the baseline comparison (Table 1) were included in the analysis. The results of the multivariate analysis were showed in Table 3. The statistic difference remained significant in history of hypertension (OR =5.27, 95% CI, 2.35, 11.85, P<0.001) and IMPD over 1.79 cm (OR =3.16, 95% CI, 1.42, 7.03, P=0.005). So the multivariate analysis supported the dilated IMPD as a novelly-identified independent risk factor of anastomotic leakage.
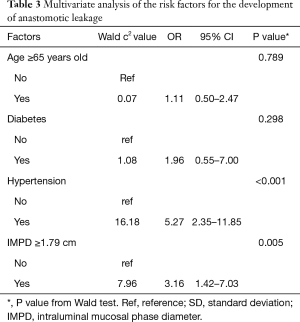
Full table
Factors correlated to the dilated IMPD
To identify the factors which might be correlated to the IMPD, we further applied a binary correlation analysis between dilated IMPD and other factors including age, gender, BMI, history of diabetes, history of hypertension, history of smoking, alcohol consumption history, duration of dysphagia, neoadjuvant therapy, maximum tumor size, and pathologic stage. Five factors presented statistically significant correlation with the dilated IMPD (Table 4). Male patients tended to have a greater IMPD (P=0.001, Spearman’s correlation coefficient =0.153). And duration of dysphagia (P=0.027, Spearman’s correlation coefficient =0.106), maximum tumor size (P<0.001, Spearman’s correlation coefficient =0.169) and pathologic stage (P=0.002, Spearman’s correlation coefficient =0.149) had a positive correlation with the dilated IMPD. Age had a negative correlation with the dilation of IMPD (P=0.042, Spearman’s correlation coefficient =−0.098).
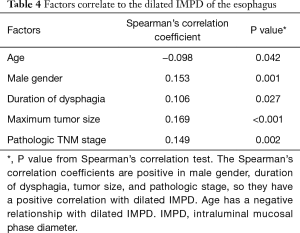
Full table
Discussion
Our study, for the first time, demonstrated that the dilation of IMPD was a novel risk factor of anastomotic leakage after intrathoracic anastomosis using a 25 mm circular stapler in patients with esophageal squamous cell carcinoma. The ROC curve indicated 1.79 cm as the cutoff value for IMPD. The leakage rate almost tripled around 1.79 cm, and the difference in leakage rate between patients with an IMPD higher than 1.79 cm and less than 1.79 cm reached statistically significant. What’s more, as the escalation of the IMPD, the leakage rate significantly increased. And in the multivariate analysis, IMPD higher than 1.79 cm also had been proved to be a risk factor of anastomotic leakage.
IFPD failed to be a risk factor in this study. IFPD was mainly affected by the amounts of barium the patients had swallowed during the barium esophagography. On the other hand, IMPD cannot significantly be affected by the amounts of barium swallowed down. So IMPD can precisely represent the natural status of the esophageal lumen. We, therefore, believed that IMPD was more reliable in judging the status of esophageal lumen than IFPD. The correlation analysis showed the dilated IMPD related to the age, gender, duration of dysphagia, maximum size of the tumor and pathologic stage closely. But in the multivariate logistic regression analysis, they were not risking factors of anastomotic leakage, indicating that IMPD was an integrated parameter of all these factors. The piled effects of these factors made the IMPD more meaningful than a single factor. Long duration of dysphagia, the large size of the tumor, and the late pathologic stage reflect the long affection of cancer on the esophagus. The accumulation of affection finally leads to the dilation of the esophagus.
Hypertension was also recognized as a risk factor of anastomotic leakage in our study. Hypertension may result in the low flow or inadequate perfusion of the anastomosis site which further delays the repairmen of the damaged esophagus (15). A retrospective study with a large sample size has reported the positive relationship between hypertension and anastomosis leakage. However, hypertension has seldom been reported independently in other studies. Most studies regard it as part of cardiovascular disease. From our results, we think hypertension as preoperative comorbidity should be reported independently.
Esophagography, although an old test, remains important in the detection of anatomic abnormities and assessment of the motility of the esophagus (11). The CT scan only provides us with the cross-section of the body, so it is unable to observe the esophagus as an entirety. This makes it hard to locate a specific site of the esophagus. The reason why we measured the diameter at 5 cm above the upper margin of the tumor has been described in the methods part. So the accurate locating is very important in the study. And the esophagus is empty when the patient undergoes the CT scan. What’s more, the patient is in a supine position during the CT scan. Under this body position the esophagus may further be compressed by the lung and mediastinum. We can only get the length of a long axis of an oval using the CT scan. Compare to the CT scan, the esophagography provides us an overall view of the esophagus when the esophagus is on functional status. It continues to be the primary radiologic modality for the evaluation of patients with dysphagia, reflux symptoms, or other clinical findings of esophagus diseases (16). Barium or other contrast swallow esophagography was routinely used in the detection of anastomotic leakage after esophagectomy in many hospitals (17,18). But the value of the examination before surgery limits to the locating of the tumor in most time. Its values in the evaluation of morphological changes of the esophagus are often underestimated by the surgeons. So does our hospital, 36.4% of patients didn’t have the barium esophagography before surgery in this study. Our findings in this study may attract the new look from thoracic surgeons at the values of the old examination in esophagus cancer patients.
The internal mechanisms why the patients with a dilated IMPD are facing a higher risk of postoperative anastomotic leakage when applying the 25 mm circular stapler are unclear now. However, this doesn’t decline the value of our findings. Our study declared patients with an intraluminal diameter over 1.79 cm were facing high risk of anastomotic leakage when applying the 25 mm circular stapler to make the gastroesophageal anastomosis. So a larger size of a circular stapler or hand-sewn anastomosis would be more suitable for these patients. The larger size of the circular stapler also could decrease the incidence anastomosis stricture (19). So our findings will push the application of larger circular stapler in patients with the dilated esophagus. What’s more, we provided a simple method to distinguish the patients with a dilated esophagus with the help of barium esophagography. However, for the patients whose diameter is normal, a large size of the circular stapler is unnecessary. The larger circular stapler is too large and challenging to be put into the esophagus. And the esophageal muscle fiber and mucosa may be torn, leading to poor blood circulation, inflammation, and hypertrophic scar formation at the site of the anastomosis.
This was a retrospective study, and the sample size in the LG was small with only 31 patients. Nonetheless, we firstly reported the dilated IMPD of the esophagus by barium esophagography as a risk factor of anastomotic leakage after intrathoracic anastomosis by 25 mm circular stapler for esophageal squamous cell carcinoma. And the method we worked out in the measure of the internal diameter of the esophagus is scientific and reliable. Prospective studies with larger sample size are needed to further test our results. The studies focus on histopathological changes of the dilated esophagus tissues or studies that apply a calibrated circular stapler of appropriate sizes to accomplish the anastomosis may finally explain the underlying mechanisms of our findings.
Acknowledgments
We would like to thank Mr. Shi-De Wu from the High School Attached to Northeast Normal University for his linguistic assistance to this manuscript.
Funding: This work was supported by the National Natural Science Foundation of China (grant number: 81672291, 31071210 to YDL).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.99). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the institutional review board of West China Hospital, Sichuan University (No. 20180321).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ajani JA, D'Amico TA, Almhanna K, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw 2015;13:194-227. [Crossref] [PubMed]
- Kataoka K, Takeuchi H, Mizusawa J, et al. A randomized Phase III trial of thoracoscopic versus open esophagectomy for thoracic esophageal cancer: Japan Clinical Oncology Group Study JCOG1409. Jpn J Clin Oncol 2016;46:174-7. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg 2017;266:232-6. [Crossref] [PubMed]
- Jeon HW, Sung SW. Minimally invasive Ivor Lewis esophagectomy for esophageal cancer. J Vis Surg 2016;2:165. [Crossref] [PubMed]
- Martin RC, Farmer RW, St Hill RC, et al. Esophageal anastomotic leak does not affect ability to receive adjuvant treatment. J Surg Oncol 2015;111:855-61. [Crossref] [PubMed]
- Cooke DT, Lin GC, Lau CL, et al. Analysis of cervical esophagogastric anastomotic leaks after transhiatal esophagectomy: risk factors, presentation, and detection. Ann Thorac Surg 2009;88:177-84; discussion 184-5. [Crossref] [PubMed]
- Gooszen JAH, Goense L, Gisbertz SS, et al. Intrathoracic versus cervical anastomosis and predictors of anastomotic leakage after oesophagectomy for cancer. Br J Surg 2018;105:552-60. [Crossref] [PubMed]
- Ohi M, Toiyama Y, Mohri Y, et al. Prevalence of anastomotic leak and the impact of indocyanine green fluorescein imaging for evaluating blood flow in the gastric conduit following esophageal cancer surgery. Esophagus 2017;14:351-9. [Crossref] [PubMed]
- Baker ME, Einstein DM. Barium esophagram: does it have a role in gastroesophageal reflux disease? Gastroenterol Clin North Am 2014;43:47-68. [Crossref] [PubMed]
- O'Rourke AK, Lazar A, Murphy B, et al. Utility of Esophagram versus High-Resolution Manometry in the Detection of Esophageal Dysmotility. Otolaryngol Head Neck Surg 2016;154:888-91. [Crossref] [PubMed]
- Katzka DA. The role of barium esophagography in an endoscopy world. Gastrointest Endosc Clin N Am 2014;24:563-80. [Crossref] [PubMed]
- Deng HY, Alai G, Luo J, et al. Cancerous esophageal stenosis before treatment was significantly correlated to poor prognosis of patients with esophageal cancer: a meta-analysis. J Thorac Dis 2018;10:4212-9. [Crossref] [PubMed]
- Ti TK, Rauff A, Goh HS. Anterior resection using the circular stapling instrument: a Singapore experience. Aust N Z J Surg 1986;56:919-22. [Crossref] [PubMed]
- Rice TW, Ishwaran H, Ferguson MK, et al. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. J Thorac Oncol 2017;12:36-42.
- Kassis ES, Kosinski AS, Ross P Jr, et al. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg 2013;96:1919-26. [Crossref] [PubMed]
- Levine MS, Rubesin SE. Diseases of the esophagus: diagnosis with esophagography. Radiology 2005;237:414-27. [Crossref] [PubMed]
- Boone J, Rinkes IB, van Leeuwen M, et al. Diagnostic value of routine aqueous contrast swallow examination after oesophagectomy for detecting leakage of the cervical oesophagogastric anastomosis. ANZ J Surg 2008;78:784-90. [Crossref] [PubMed]
- Solomon DG, Sasaki CT, Salem RR. An evaluation of the routine use of contrast radiography as a screening test for cervical anastomotic integrity after esophagectomy. Am J Surg 2012;203:467-71. [Crossref] [PubMed]
- Hosoi T, Abe T, Uemura N, et al. The Impact of Circular Stapler Size on the Incidence of Cervical Anastomotic Stricture After Esophagectomy. World J Surg 2019;43:1746-55. [Crossref] [PubMed]


