
CAR-T cell therapy for lung cancer: a promising but challenging future
Lung cancer is one of the most commonly diagnosed cancers and the leading cause of cancer death. For many years, the main treatments for lung cancer include surgery, chemotherapy, radiotherapy and targeted therapy. Recently immunotherapy, in particular, the programmed death 1 (PD-1) inhibitors, has become the first-line therapy for lung cancer (1). The emergence of chimeric antigen receptor (CAR)-T cell immunotherapy also provides a new approach and new hope for the treatment of lung cancer. However, the challenges for CAR-T cell therapy in eradicating solid tumors are immense (2). Currently, there are more than 250 clinical trials worldwide evaluating the safety and efficacy of CAR-T cell therapy in the treatment of solid tumors. China and the United States have the largest number of CAR-T clinical trials (3). This paper summarizes some of the recent results for lung cancer treatment and discusses the many challenges and problems we still face in translating these new CAR-T therapies into the clinic to treat lung cancer patients. These challenges include, improvement in the flexibility of the CAR structure, more specificity in tumor antigen targeting, overcoming the complexities of the hostile lung tumor microenvironment (TME), and in many cases the accessibility and penetration of the large tumor volume for effective treatment.
Evolution of the CAR structure
From initial conception the use of CARs in T-cell therapy has undergone four progressive generations generically based on the intracellular signal domains of the CAR (2). The first generation of CARs, containing only the antigen recognition signal, had poor activity and a short survival time in vivo (4). The design of the second and third generation CARs included one and two costimulatory molecules within the signal transduction region, respectively. These modifications were designed to enhance T cell proliferation, cytotoxic, and prolonged T cells’ survival time. The optimization of the co-stimulatory molecules in the CARs led to enhanced CAR-T cell function. Most commonly used second-generation co-stimulatory domains are 4-1BB or CD28. DNAX-activating protein 10 (DAP10) has also been shown to enhanced cytotoxicity, cytokine secretion and T cell activation. In in vivo mouse models of human lung cancer xenotransplantation, delayed growth of primary lung cancer and improved anti-tumor efficacy were observed based on non-small cell lung cancer (NSCLC) cell lines (5). The fourth generation CAR-T design introduced pro-inflammatory cytokines and co-stimulating ligands, to enhance the ability of the T-cells to penetrate and overcome the suppressive nature of the hostile TME (2).
In addition to the intracellular signal transduction modules, the improvement of the extracellular module structure has also been shown to improve the amplification and anti-tumor efficacy of CAR-T cells. Qin et al. proposed that the incorporation of a hinge structure improved the flexibility of the single-chain variable fragment (scFv) which binds and promotes the expansion, migration and invasion of cluster of differentiation 4 (CD4)+ CAR-T cells (6). The structural design of the CARs is continuously being optimized and key in the efficacy of CAR-T, although the second-generation CAR-T cells still remains the mainstream approach for therapeutic application.
Antigenic heterogeneity and specific targeting of NSCLC
The ideal target for CAR-T cell therapy is when the target-antigen is only expressed on cancer cells or overexpressed on all or most lung cancer cells compared to normal cells. Although many tumor-associated antigens (TAA) have been detected in NSCLCs (7), CAR-T cells have been designed to target only a small number of these antigens (8). At the same time, some of these target-antigens are also expressed in low amounts in normal tissues, thus some CAR-T cells have the potential to attack normal cells.
Targets currently under evaluation for CAR-T cell therapy for lung cancer include: epidermal growth factor receptor (EGFR); human epidermal growth factor receptor 2 (HER2); mesothelin (MSLN); prostate stem cell antigen (PSCA); mucin 1 (MUC1); carcinoembryonic antigen (CEA); tyrosine kinase-like orphan receptor 1 (ROR1); programmed death ligand 1 (PD-L1) and CD80/CD86. Table 1 lists the current clinical research targets and clinical trials of CAR-T cell therapy for lung cancer.
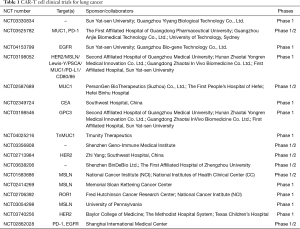
Full table
EGFR is expressed in both epithelial cells and many epithelium-derived malignancies. Compared to normal lung tissues, the significant elevation of affinity of binding sites in lung carcinomas makes EGFR a promising therapeutic target. The second-generation lentivirus-transduced EGFR-CAR-T cells proved to be safe and a feasible option for patients with EGFR-positive (>50% expression), relapsed/refractory NSCLCs in a phase I clinical study (NCT01869166) (9).
HER2 is also a potential CAR-target antigen in lung cancer. Generally, HER2-targeted CAR-T cells have demonstrated good therapeutic benefits in patients with recurrent/refractory HER2-positive sarcomas with no observed respiratory distress after treatment. However, in one case study, a patient, with metastatic colon cancer migrating to the lungs and liver, experienced respiratory distress within 15 minutes after 1×1010 HER2-targeted CAR-T cells infusion. Morgan et al. speculated that it was related to low levels of HER2 expression on the normal lung epithelial cells, which may have caused an auto-immune response (10). Thus, the safety and efficacy of HER2-targeted CAR-T may be compromised in the treatment of some lung cancer patients depending on the HER2 expression. Therefore, although HER2 is generally considered a strong candidate-target, the cause of respiratory distress caused by HER2-targeted CAR-T, albeit not common exemplifies the need to understand tumor characteristics and design of alternative specific-antigen targets.
In a different study, the lung cancer target, MSLN, was shown to be expressed in 69% of lung adenocarcinoma. One in five adenocarcinoma patients strongly expressing MSLN, with no MSLN expression detected in normal lung tissue (11). MSLN CAR-T cell therapy reduced the tumor burden in pre-clinical mouse models (12).
The expression of MUC1, a transmembrane glycoprotein, is aberrantly upregulated in NSCLC. PSCA is a glycosylphosphatidylinositol (GPI)-anchored cell surface antigen that is also frequently overexpressed in NSCLC. The design of combinational CAR-PSCA and CAR-MUC1-T cells, as proposed by Wei et al., showed excellent anti-NSCLC efficacy compared with the treatment of CAR-T cells targeting a single antigen (13). The study demonstrates that PSCA and MUC1 are both promising CAR-T cell targets in NSCLC. CEA is overexpressed in nearly 70% of NSCLCs (14). However, some patients who received CAR-T cell therapy targeting CEA, had transient, acute respiratory toxicity. Expression of CEACAM5 on lung epithelium cells has been proposed as a mechanism that may have contributed to this transient toxicity (15). It suggests that methods to control CAR-T ‘on-target, off-tissue’ toxicity are required to enable a clinical impact of this approach in solid malignancies. ROR1 exhibits high and homogeneous cell surface expression in many epithelial tumors and some B cell malignancies. However, ROR1 was expressed in some normal tissues, raising concerns that targeting ROR1 in patients may cause toxicity. To improve selectivity, Srivastava et al. creatively engineered T cells with synthetic Notch (synNotch) receptors specific for EpCAM or B7-H3, which are expressed on ROR1+ tumor cells but not ROR1+ stromal cells. SynNotch receptors induced ROR1 CAR expression selectively within the tumor, resulting in tumor regression without toxicity (16). CD80/86 are costimulatory molecules of the immune cells. Binding of CD80/CD86 to CTLA-4 can lead to downregulation of T cell function through a variety of mechanisms. The central role of the CTLA4-CD80/CD86 pathway in co-stimulation makes it a preferred target for immune intervention (17). CD80/CD86 mRNA expression has been detected in a large number of NSCLC cell lines (18). As CD80/CD86 is also expressed in normal immune cells, there is a risk of developing autoimmunity. New strategies are expected to be developed to enable CD80/CD86 CAR-T cells to differentiate between normal cells and tumor cells.
In summary, EGFR, MSLN and multi-targeted combinations may be more suitable targets in the treatment of lung cancer in the light of HER2, CEA and ROR1 CAR-T cells causing serious adverse reactions in some patients and CD80/CD86 CAR-T may induce autoimmunity.
Immune microenvironment and checkpoint inhibitors
To evade attack from the immune system tumor cells have developed an evasion strategy. The immune system is in constant surveillance. When T cells are activated they express immune checkpoint proteins, such as the PD-1 on the cell surface which binds to its ligand (PD-L1) expressed on the surface of host cells to prevent a host autoimmune reaction. Tumor cells express the PD-1 ligand (PD-L1 or PD-L2) and by binding to PD-1 on the T cell they evade immune cell recognition and attack from the immune system (2). Blocking the interaction between PD-1 and PD-L1 to allow the T cells to recognise cancer cells and to enhance immune function is now being utilized as an anti-tumor therapy and a promising strategy for the treatment of lung cancer.
The use of PD-1 and PD-L1 monoclonal antibodies (mAbs) to block the PD-1-PD-L1 interaction as a cancer therapy has FDA approval and have been in clinical use for a number of years (1). Another effective approach to block the PD-1/PD-L1 interaction is through the design of CAR-T cells engineered to secrete the checkpoint PD-1 inhibitor. Rafiq et al. demonstrated that CAR-T cells with scFv secreting PD-1 enhanced the survival rate of PD-L1 (+) tumor-bearing mice in both homogenous and xenograft mouse models, acting through autocrine and paracrine mechanisms (19). This strategic approach enhanced the efficacy of CAR-T cells in cancers within the immunosuppressive microenvironment. Our group, Chen et al., successfully applied the combination of CAR-T cells and PD-1 knockout in the clinical treatment of lung cancer. The clinical trial (NCT03525782) indicated that the treatment was safe, but the therapeutic effect varied greatly depending on the individual patient. Factors influencing the variation in clinical outcomes are currently under investigation (20).
Problems with CAR-T cells infiltration into solid tumor tissue
Infiltration of CAR-T cells into solid tumor tissues is a prerequisite for their anti-tumor function, which relies on their efficient and specific trafficking capabilities. Mismatching of chemokine-chemokine receptor pairs, down-regulation of adhesion molecules, aberrant vasculature, the immunosuppressive TME and anatomical location of immune effector cells, may all contribute to the poor homing of these cells (21). To overcome the problems associated with the CAR-T cells entering into the solid tumor environment or penetrating the extracellular matrix (ECM) of the tumor, Caruana et al. modified CAR-T cells to express heparinase (HPSE), an enzyme that aids in the degradation of the tumor ECM components, and hence promote T-cell invasion and anti-tumor activity (22). Another approach to successfully infiltrate large solid tumors in the lung was developed by Hu et al., where they co-administered interleukin 12 (IL-12) DNA and the chemotherapy drug doxorubicin before CAR-T cell infusion (23). The combination of IL-12 plus doxorubicin not only promoted NKG2D (+) CD8(+) T cell infiltration into large solid tumors in the mouse lung cancer model, but also co-up-regulated the production of chemokines CXCL9 and CXCL10 that attracted T cells. Thus, the accumulation of T cells in the tumor microenvironment was promoted, and the effector function of infiltrating T cells was enhanced by increasing the ratio of the stimulator and regulator. Intrapleural administration of CAR-T cells enabled more effective infiltration of T cells into the tumor microenvironment, requiring 30 times fewer CAR-T cells than systemic intravenous administration. These CAR-T cells rapidly expanded and differentiated, and induced long-term remission of tumors, and regional T cell administration also promoted effective elimination of tumors outside the thoracic cavity (24).
T cell exhaustion
T cells infiltrating into lung tumors is also affected by a phenomenon known as T cell exhaustion. A recent study by Chen et al. found that a transcription factor family called NR4A played an important role in T cell exhaustion, and these transcription factors were shown to limit CAR-T cell function in solid tumors (25). Using mouse models, they demonstrated that CAR-T cells function more effectively when NR4A transcription factors were lacking, reducing tumor size and increasing the survival rate of mice with cancer.
Although these findings have not been directly applied to clinical studies of CAR-T therapy for lung cancer, analyzing the role of NFAT and NR4A transcription factors solves an immunological mystery and provides scientists with new clues for designing better anti-tumor strategies. NR4A enriched in CD8 + PD-1hi TILs in NSCLC (25), so blocking NR4A, may also be a promising treatment for NSCLC.
In summary, the clinical application of CAR-T in lung cancer treatment is still undergoing extensive research. However, the continuous improvement of CAR-T technology for lung cancer is providing much promise but many challenges. Although the toxicology results are favorable, we still face many generic challenges before using CAR-T based therapy as a viable alternative, or as an adjunct treatment for lung cancers. Future efforts are being made to find more specific target antigens for lung cancer cells to reduce adverse side effects, as well as continuously optimization of CAR-T cells through improvement in genetic engineering, enabling an increase in the number of CAR-T cells that migrate to tumor sites and enhance the anti-lung cancer ability.
Acknowledgments
We are grateful to the useful suggestions given by Dr. Eileen McGowan and Dr. Yiguang Lin (University of Technology Sydney).
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Peng Luo, Clare Y. Slaney and Jian Zhang) for the series “Immunotherapy and Tumor Microenvironment” published in Journal of Thoracic Disease. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.118). The series “Immunotherapy and Tumor Microenvironment” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sui H, Ma N, Wang Y, et al. Anti-PD-1/PD-L1 therapy for non-small-cell lung cancer: toward personalized medicine and combination strategies. J Immunol Res 2018;2018:6984948.
- McGowan E, Lin Q, Ma G, et al. PD-1 disrupted CAR-T cells in the treatment of solid tumors: Promises and challenges. Biomed Pharmacother 2020;121:109625. [Crossref] [PubMed]
- June CH, O’Connor RS, Kawalekar OU, et al. CAR T cell immunotherapy for human cancer. Science 2018;359:1361-5. [Crossref] [PubMed]
- Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res 2006;12:6106-15. [Crossref] [PubMed]
- Zhao R, Cheng L, Jiang Z, et al. DNAX-activating protein 10 co-stimulation enhances the anti-tumor efficacy of chimeric antigen receptor T cells. Oncoimmunology 2019;8:e1509173. [Crossref] [PubMed]
- Qin L, Lai Y, Zhao R, et al. Incorporation of a hinge domain improves the expansion of chimeric antigen receptor T cells. J Hematol Oncol 2017;10:68. [Crossref] [PubMed]
- Djureinovic D, Hallstrom BM, Horie M, et al. Profiling cancer testis antigens in non-small-cell lung cancer. JCI Insight 2016;1:e86837. [Crossref] [PubMed]
- Zeltsman M, Dozier J, McGee E, et al. CAR T-cell therapy for lung cancer and malignant pleural mesothelioma. Transl Res 2017;187:1-10. [Crossref] [PubMed]
- Feng K, Guo Y, Dai H, et al. Chimeric antigen receptor-modified T cells for the immunotherapy of patients with EGFR-expressing advanced relapsed/refractory non-small cell lung cancer. Sci China Life Sci 2016;59:468-79. [Crossref] [PubMed]
- Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 2010;18:843-51. [Crossref] [PubMed]
- Kachala SS, Bograd AJ, Villena-Vargas J, et al. Mesothelin overexpression is a marker of tumor aggressiveness and is associated with reduced recurrence-free and overall survival in early-stage lung adenocarcinoma. Clin Cancer Res 2014;20:1020-8. [Crossref] [PubMed]
- Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A 2009;106:3360-5. [Crossref] [PubMed]
- Wei X, Lai Y, Li J, et al. PSCA and MUC1 in non-small-cell lung cancer as targets of chimeric antigen receptor T cells. Oncoimmunology 2017;6:e1284722. [Crossref] [PubMed]
- Berinstein NL. Carcinoembryonic antigen as a target for therapeutic anticancer vaccines: a review. J Clin Oncol 2002;20:2197-207. [Crossref] [PubMed]
- Thistlethwaite FC, Gilham DE, Guest RD, et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol Immunother 2017;66:1425-36. [Crossref] [PubMed]
- Srivastava S, Salter AI, Liggitt D, et al. Logic-gated ROR1 chimeric antigen receptor expression rescues T cell-mediated toxicity to normal tissues and enables selective tumor targeting. Cancer Cell 2019;35:489-503.e8. [Crossref] [PubMed]
- Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol 2002;3:611-8. [Crossref] [PubMed]
- Wroblewski JM, Bixby DL, Borowski C, et al. Characterization of human non-small cell lung cancer (NSCLC) cell lines for expression of MHC, co-stimulatory molecules and tumor-associated antigens. Lung Cancer 2001;33:181-94. [Crossref] [PubMed]
- Rafiq S, Yeku OO, Jackson HJ, et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol 2018;36:847-56. [Crossref] [PubMed]
- Chen S, Lin Y, Zhong S, et al. 33O Anti-MUC1 CAR-T cells combined with PD-1 knockout engineered T cells for patients with non-small cell lung cancer (NSCLC): A pilot study. Ann Oncol 2018;29:mdy485.002.
- Slaney CY, Kershaw MH, Darcy PK. Trafficking of T cells into tumors. Cancer Res 2014;74:7168-74. [Crossref] [PubMed]
- Caruana I, Savoldo B, Hoyos V, et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat Med 2015;21:524-9. [Crossref] [PubMed]
- Hu J, Sun C, Bernatchez C, et al. T-cell homing therapy for reducing regulatory T cells and preserving effector T-cell function in large solid tumors. Clin Cancer Res 2018;24:2920-34. [Crossref] [PubMed]
- Adusumilli PS, Cherkassky L, Villena-Vargas J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med 2014;6:261ra151. [Crossref] [PubMed]
- Chen J, Lopez-Moyado IF, Seo H, et al. NR4A transcription factors limit CAR T cell function in solid tumours. Nature 2019;567:530-4. [Crossref] [PubMed]


