
Cone beam navigation bronchoscopy: the next frontier
Introduction
Lung cancer accounts for close to a quarter of all cancer-related death in United States (1). Five-year survival in lung cancer varies greatly based on the stage of disease at diagnosis, ranging from 55% in localized disease to 4% in metastatic disease (2). Early lung cancer diagnosis through screening with low-dose computed tomography demonstrated a 20% reduction in lung cancer mortality in current or former smokers with a history of 30 or more pack-years (3). These results led to significant efforts in early detection and early treatment of lung cancer. As such, peripheral lung nodule diagnosis and management have become key elements in the practice of pulmonary medicine.
Peripheral pulmonary lesions (PPLs) create significant diagnostic and therapeutic challenges for pulmonologists. Conventional bronchoscopy using transbronchial biopsies (TBBX) or transbronchial needle aspiration (TBNA) have a low sensitivity (14–63%) for diagnosing malignant lesions (4-6). The presence of bronchus sign on CT scan is a predictor of higher diagnostic yield (DY). A recent meta-analysis reported 74.1% versus 49.6% yield in lesions with versus without bronchus sign, respectively (7). Moreover, a recent multicenter prospective randomized trial showed that the DY was only 37% in conventional bronchoscopy with fluoroscopy for PPLs (8). CT-guided transthoracic needle biopsy of PPLs has a higher sensitivity (up to 90%) compared with conventional bronchoscopy, but it is associated with higher complication rates (risk of pneumothorax is around 25% in some studies), does not allow biopsies of multiple targets or concurrent mediastinal staging as is possible with bronchoscopic approaches (9).
Electromagnetic navigation bronchoscopy (ENB) is an image-guided technique for biopsy of PPLs. A meta-analysis including 15 trials demonstrated that the DY of ENB was 64.9% (95% CI, 59.2–70.3) (10). Although ENB is often referred to as a guidance system for PPLs biopsy, ENB is not a real-time technique. In order to improve DY of PPLs further, ENB can be combined with other modalities, such as radial probe endobronchial ultrasound (RP-EBUS), cone-beam computed tomography (CBCT), ultrathin bronchoscopy, or confocal endomicroscopy. This article reviews the techniques and diagnostic capabilities with CBCT-guided ENB for diagnosis of PPLs and describes promising clinical observations and innovations in this area.
CBCT
While CBCT has been widely used in interventional radiology, neurosurgery, vascular surgery, and interventional cardiology, it only recently has been applied to the field of interventional pulmonology (11). C-arm CBCT employs a flat panel detector system made from cesium iodide (CsI) scintillators to produce CT-like cross sectional multiplanar and three-dimensional (3D) images. Image acquisition involves the projection of a cone-shaped (wide collimation) X-ray beam from the X-ray source onto the flat panel detector. With CBCT, a complete data volume set is acquired in one single rotation of the source and detector around the patient. In contrast, a conventional CT employs a fan-shaped X-ray beam (narrow collimation) that requires numerous revolutions in sequential fashion to acquire the same data volume set from the patient (12).
Since the first CBCT performed at Mayo Clinic Biodynamics Research Laboratory in 1982, there has been significant advancement in systems hardware and software. Today, CBCT image resolution is approaching that of conventional CT. Usually a ceiling- or floor-mounted C-arm format, CBCT scans usually takes 4 to 10 seconds to perform and can offer standard fluoroscopy capabilities, which make this tool a valuable addition to bronchoscopy. The main advantages of CBCT compared to multi-detector CT (MDCT) are: (I) lower radiation dose, (II) decreased time of image acquisition, and (III) decreased patient movement interference. Additionally, the ability to perform real-time multiplanar confirmation with 3D reconstruction is useful to confirm accurate biopsy tool position within the target lesion (13).
In clinical practice, CBCT is typically used in addition to navigation bronchoscopy. Patient is intubated and sedated. An initial CBCT scan is then performed. Segmentation of the targeted lung nodule is done on the acquired CT volume and then projected onto the standard fluoroscopy—this is referred to as AF. The bronchoscopist will then perform navigation bronchoscopy to the target, which is made readily visible with AF. The projected lesion can be viewed in any fluoroscopy viewing angle with respect to biopsy tools. Verification of catheter to target should be done in at least two orthogonal viewing angles to ensure properly catheter target alignment. This ability to both accurately localizes the lesion as well as real time confirmation of biopsy tools in lesion affords more confidence in navigation bronchoscopy and biopsy. Additionally, lesions that are not visible on fluoroscopy, such as groundglass opacities or cystic lesions, can now be visible via AF (Figures 1,2). Currently, Philips Azurion Lung Suite is a commercial package designed to streamline this process and allow seamless usage with navigation bronchoscopy.
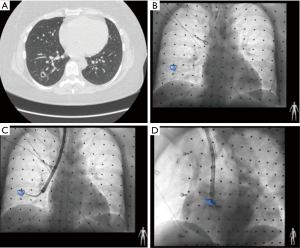
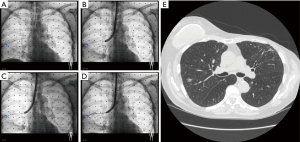
Navigation bronchoscopy—clinical challenges
When approaching navigation bronchoscopy, navigation platforms aim to extend the bronchoscopist’s anatomical knowledge and abilities. Regardless of the commercially available system, all navigation platforms [i.e., EMB, AF, Virtual Bronchoscopy, Robotic Bronchoscopy] begin with the same baseline—a thin-cut chest CT scan with patient at the end of an inspiratory hold maneuver with lung volume close to total lung capacity (TLC). This is done to enhance airway segmentation on the planning software so to afford better navigation path selection. Some system attempts to use a second CT chest scan at end-expiration in order to account of respiratory phasic variations. Nonetheless, the lung volume of the CT scan used at the planning is very different than the actual patient lung volume at the time of the procedure. This creates a significant clinical problem in navigation bronchoscopy: CT-to-Body divergence (CTBD).
CTBD is the difference between PPL location at the time of planning CT and at the time of procedure. There are multiple factors influencing the degree of CTBD. One of the major contributors is the patient lung volume at the time of the planning CT and the patient lung volume at the time of the procedure. While there are variations in ventilation and sedation strategies during EMB, increasingly navigation procedures are done with patient intubated, sedated and paralyzed. Assuming the lung volume at the time of planning CT is close to TLC, then the lung volume at the procedure is closer to functional residual capacity (FRC). CTBD is further amplified by increased patient body habitus, the inherent obstruction of endotracheal tube by the bronchoscope, fouling of the airways with mucus or blood, regional collapse of the target lobe due to over-wedging of the bronchoscope, diaphragm motion (especially in the lower lobes), and post-biopsy hemorrhage at the location of PPL. These considerations should be weighed when performing navigation bronchoscopy. Due to the increasing awareness of the importance of CTBD, some bronchoscopists are performing navigation procedure prior to lymph node staging with EBUS. The intention is to reduce atelectasis that can occur during EBUS biopsy procedure.
A second challenge is the Tool-in-Catheter deflection (TICD) that occurs when the biopsy instrument causes substantive alteration of the position of the working catheter through which the instrument is being passed. Additionally, working catheter selection can influence the targeting accuracy as exaggerated catheter curvature can cause distortions of lung parenchyma (i.e., an emphysematous lung may be pulled and distorted with a highly curved catheter). In order to solve this problem, there have been significant efforts in developing directable catheters, flexible biopsy tools (mostly needles), and more recently robotic bronchoscopy platforms for stable catheter positioning and direction. Yet, these efforts do not address CTBD nor the issue of real-time imaging confirmation.
CBCT overcoming navigation bronchoscopy challenges
Real-time confirmation of tool-in-lesion holds the potential to overcome the above mentioned navigation bronchoscopy challenges. Currently, fluoroscopy and radial EBUS provide real-time confirmation of catheter relationship to the PPL. However, due to the technical challenges described above, navigation success often does not equate to positive biopsy yield. When using navigation systems that provide a pathway to the target lesion, accurate real-time confirmation of tool-in-lesion may hold the key to increase confidence in adequately biopsied material and thus DY. The tip of biopsy instruments often deflect the catheter or overlap the target in a plane perpendicular to the fluoroscopic view, resulting in perceived navigation success but yielding negative biopsy samples. CBCT reduces this possibility of falsely perceived navigation success. Moreover, if there are intra-tumor metabolic heterogeneity seen on PET-CT scans, this may lead to targeted sampling of specific portions of the PPL that have the most metabolic activity. CBCT also permits this selectively targeted biopsy. Finally, CBCT can detect atelectasis which is often not readily visible on fluoroscopy; as such, CBCT can help to guide ventilation strategies aimed at reducing atelectasis and for better visualization of targeted lesions, particularly at the lung bases (14).
Review of evidence
Data on CBCT-guided navigation bronchoscopy for PPLs are limited. Until recently, CBCT-enabled AF image guidance were performed in hybrid operating rooms or interventional radiology suites via transthoracic needle aspiration (15,16). In the pulmonary space, recent studies often utilize a combination of modalities such as ENB, CBCT, R-EBUS, AF, ultrathin bronchoscope (Table 1). In the largest study to date, Pritchett et al. described their experience utilizing intraprocedural CBCT with AF during ENB-guided biopsy of peripheral lung nodules (17). A total of 93 lesions with a median size of 16.0 mm were biopsied in 75 consecutive patients. The DY was 83.7% (95% confidence interval, 74.8–89.9%). The only complication was pneumothorax in 4%. It is worth mentioning that R-EBUS was not used in all cases in this retrospective study. Interestingly, they found no independent correlation between lesion size, lesion location, lesion visibility under standard fluoroscopy, and the presence of a bronchus sign with DY after multivariate regression analysis. A smaller retrospective study by Sobieszczyk et al. reported the use of combination of CBCT, ENB, R-EBUS with or without Trans Bronchial Access Tool (TBAT) to biopsy PPLs with DY of 77.2% (18). Comparatively, Bowling et al. reported the DY of 71% with similar combination of modalities including CBCT, ENB and TBAT without R-EBUS (19). A prospective pilot study by Casal et al. reported ODY of 70% with CBCT-guided ultrathin bronchoscope for diagnosis PPLs (20). Additionally, Ali et al. reported a DY of 90% when utilizing CBCT-guided ultrathin bronchoscope and virtual bronchoscopic navigation (VBN) (21). In this study, the authors were able to guide the biopsy forceps within or adjacent to the target lesions under CBCT guidance in 95.0% of patients. Multiple factors contribute to DY such as size, lesion location, procedural elements, and patient factors. More comparative and prospective studies are needed to evaluate CBCT combined navigation for diagnosis PPLs in the future to delineate the best clinical practice and combination of techniques.
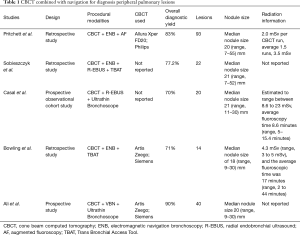
Full table
Future directions
As innovation continues to drive the development of new tools and methodologies for access to peripheral nodule, several questions need consideration. First, is there a dominant methodology in peripheral nodule access and enabling future therapeutic modalities such as peripheral nodule ablation? Given the crowded navigation bronchoscopy space, it is hard to determine which modality or combination of modalities will contribute the most to increase DY. Quality, comparative research is needed. Second, given the capital cost of each diagnostic modalities, when will the equipment become cost prohibitive. Third, CBCT can enable the therapeutic options for peripheral nodule. However, the effectiveness of peripheral ablation compared to surgical gold standard still remain to be determined. Fourth, several systems of CBCT are available on market today, and whether there is a difference in their performance is not clear. Finally, should future navigation studies be determined by DY (if so, is there a universal accepted definition of DY) or should the success of a navigation procedure be defined as tool-in-lesion. These questions remain to be answered by our field, and as such allow for an exciting time for further investigation. But one thing can be certain, CBCT is here to stay.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Fabien Maldonado and Robert Lentz) for the series “Novel Diagnostic Techniques for Lung Cancer” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.85). The series “Novel Diagnostic Techniques for Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. GZC has served as a consultant to Boston Scientific; Medtronic plc; Pinnacle Biologics, Inc.; and Restor3D and has received research funding from Intuitive Surgical Inc. and Pinnacle Biologics, Inc. MW served as a consultant to Boston Scientific; Nuvaira Inc.; Olympus Corporation; and Veracyte, Inc. and has served as a reviewer on the Data Safety Monitoring Board for CSA Medical Inc. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclaimer: The views expressed in this article reflect the results of research conducted by the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Lung Cancer Fact Sheet. American Lung Association. 2019. Available online: https://www.lung.org/lung-health-and-diseases/lung-disease-lookup/lung-cancer/resource-library/lung-cancer-fact-sheet.html . Accessed 12/23 2019.
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Rivera MP, Mehta AC, American College of Chest P. Initial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:131S-48S.
- Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e142S-65S.
- Baaklini WA, Reinoso MA, Gorin AB, et al. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000;117:1049-54. [Crossref] [PubMed]
- Ali MS, Sethi J, Taneja A, et al. Computed Tomography Bronchus Sign and the Diagnostic Yield of Guided Bronchoscopy for Peripheral Pulmonary Lesions. A Systematic Review and Meta-Analysis. Ann Am Thorac Soc 2018;15:978-87. [Crossref] [PubMed]
- Tanner NT, Yarmus L, Chen A, et al. Standard Bronchoscopy With Fluoroscopy vs Thin Bronchoscopy and Radial Endobronchial Ultrasound for Biopsy of Pulmonary Lesions: A Multicenter, Prospective, Randomized Trial. Chest 2018;154:1035-43. [Crossref] [PubMed]
- Heerink WJ, de Bock GH, de Jonge GJ, et al. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol 2017;27:138-48. [Crossref] [PubMed]
- Gex G, Pralong JA, Combescure C, et al. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: a systematic review and meta-analysis. Respiration 2014;87:165-76. [Crossref] [PubMed]
- Orth RC, Wallace MJ, Kuo MD, et al. C-arm cone-beam CT: general principles and technical considerations for use in interventional radiology. J Vasc Interv Radiol 2008;19:814-20. [Crossref] [PubMed]
- Orth RC, Wallace MJ, Kuo MD, et al. C-arm cone-beam CT: general principles and technical considerations for use in interventional radiology. J Vasc Interv Radiol 2009;20:S538-44. [Crossref] [PubMed]
- Krimsky WS, Pritchett MA, Lau KKW. Towards an optimization of bronchoscopic approaches to the diagnosis and treatment of the pulmonary nodules: a review. J Thorac Dis 2018;10:S1637-44. [Crossref] [PubMed]
- Ishiwata T, Gregor A, Inage T, et al. Advances in interventional diagnostic bronchoscopy for peripheral pulmonary lesions. Expert Rev Respir Med 2019;13:885-97. [Crossref] [PubMed]
- Rotolo N, Floridi C, Imperatori A, et al. Comparison of cone-beam CT-guided and CT fluoroscopy-guided transthoracic needle biopsy of lung nodules. Eur Radiol 2016;26:381-9. [Crossref] [PubMed]
- Abi-Jaoudeh N, Fisher T, Jacobus J, et al. Prospective Randomized Trial for Image-Guided Biopsy Using Cone-Beam CT Navigation Compared with Conventional CT. J Vasc Interv Radiol 2016;27:1342-9. [Crossref] [PubMed]
- Pritchett MA, Schampaert S, de Groot JAH, et al. Cone-Beam CT With Augmented Fluoroscopy Combined With Electromagnetic Navigation Bronchoscopy for Biopsy of Pulmonary Nodules. J Bronchology Interv Pulmonol 2018;25:274-82. [Crossref] [PubMed]
- Sobieszczyk MJ, Yuan Z, Li W, et al. Biopsy of peripheral lung nodules utilizing cone beam computer tomography with and without trans bronchial access tool: a retrospective analysis. J Thorac Dis 2018;10:5953-9. [Crossref] [PubMed]
- Bowling MR, Brown C, Anciano CJ. Feasibility and Safety of the Transbronchial Access Tool for Peripheral Pulmonary Nodule and Mass. Ann Thorac Surg 2017;104:443-9. [Crossref] [PubMed]
- Casal RF, Sarkiss M, Jones AK, et al. Cone beam computed tomography-guided thin/ultrathin bronchoscopy for diagnosis of peripheral lung nodules: a prospective pilot study. J Thorac Dis 2018;10:6950-9. [Crossref] [PubMed]
- Ali EAA, Takizawa H, Kawakita N, et al. Transbronchial Biopsy Using an Ultrathin Bronchoscope Guided by Cone-Beam Computed Tomography and Virtual Bronchoscopic Navigation in the Diagnosis of Pulmonary Nodules. Respiration 2019;98:321-8. [Crossref] [PubMed]


