
Scoring tools to monitor risk of disease progression in patients with cystic fibrosis
Cystic fibrosis (CF) is a genetic, autosomal recessive, chronic, and progressive disease, leading to clinical manifestations in multiple organ systems, including the lungs, intestines, and pancreas (1). The disease is caused by genetic mutations in the long arm of chromosome 7, which encodes the protein cystic fibrosis transmembrane regulator (CFTR), a chloride channel at the epithelial cell surface that allows co-transport of sodium and chloride along with water across the cell membrane (2). More than 1,500 mutations have been identified with the most common gene mutation, F508del, occurring in approximately 70% of the CF population (3).
The development of the disease is characterized by progressive pulmonary deterioration resulting from inflammation, retention of mucus, chronic infection by Staphylococcus aureus and Pseudomonas aeruginosa, airway obstruction and hyperinflation (4). Moreover, periodic exacerbations also contributes to decrease pulmonary function (5). Therefore, the impairment of lung function, quantified by measuring the forced expiratory volume in the first second (FEV1), expressed as a percentage of the predicted, is one of the main markers influencing clinical decisions regarding treatment management, intensification of therapeutic plans and indication of lung transplant in patients with CF (6,7). Although morbidity and mortality are still an important issue in the disease management, life expectancy is increasing consistently through the past decades with advancement in the diagnosis and treatment. Cystic Fibrosis Foundation data shows that the expected median survival in the year 2015 was 41.7 years (8).
CF management guidelines recommends the regular monitoring of patients by routine clinic visits and annual review, including the evaluation of respiratory disease/exacerbation, nutrition and digestive problems, exercise and psychological health (9). Regarding respiratory disease/exacerbation, lung function should be measured at every clinic visit and upper respiratory tract samples should be taken for microbiological analysis. Nevertheless, accurate and objective classification of disease severity and progression is still an important issue in the management of patients with CF. Therefore, cystic fibrosis severity scores have been used for decades to assess the extent of lung injury, to compare clinical severity of patients, to assess the effects of therapeutic interventions, and to estimate prognosis (10). However, there is no consensus regarding the ideal score to use.
One of the first clinical scores developed for patients with CF, the Shwachman-Kulczycki (SK) has been used for decades (11). However, despite being the most used, many criticisms have arisen on its application, including the subjectivity and broadness of its criteria, as well as the need of a chest X-ray exam. In addition, it does not emphasize the evaluation of the respiratory system, as lung function tests and complications resulting from disease progression are not included (12). Since the SK score publication, many other scoring systems for different aspects of CF have been developed, predominantly radiological (13-15) and clinical (16-19). One of them is the Cystic Fibrosis clinical score, created by Kanga et al. (18) for the evaluation of acute pulmonary exacerbations. The score aims to identify the worsening of the disease, to predict improvement or worsening of respiratory function and to evaluate therapeutic effects. Similarly, another clinical score, simple and easy to use, is the CF-ABLE score (19). It uses clinical parameters (FEV1% predicted, number of exacerbations, age and body mass index) measured at each routine clinic visit to score in a scale from 0 to 7. Patients presenting a low score have a decreased risk of death or lung transplantation in a period of 4 years. On the other hand, individuals presenting a score higher than 5 points are associated to a 26% risk increase for a poor outcome in a period of 4 years, as the risk significantly increases as the score increases.
Thus, scoring systems may be helpful in CF management, especially to detect those patients at risk of significant pulmonary function deterioration, increasing the chances for a lung transplantation, considering that decline of lung function is the main responsible for most of fatal outcomes in CF. The study of Marsteller et al. (20) shows the development and evaluation of the cystic fibrosis risk of disease progression (CF RD-PRO) score as an instrument to identify high-risk individuals for disease advancement, with a fall of 10% or more in FEV1 predicted. The CF RD-Pro score includes the number of Staphylococcus aureus infections and the body mass index with consideration to age and sex. The study showed that subjects with CF RD-Pro scores considered as high (higher or equal to 2 points) versus low-moderate (lower than 2 points) risk were approximately ten times more likely to present important clinical disease progression. The score seems to be simple to calculate and easy to apply, contributing to CF monitoring and management, although further tests in larger CF cohorts are highly needed.
Although most of these scores are classified as clinical, the variables included, the evaluation criteria and the scoring system for each of them are usually different, which makes comparisons difficult to perform and analyze. As a common point, nutritional status assessment seems to be present in the majority of the scoring systems, reinforcing the importance of the nutritional management in the monitoring and progression of the disease. Table 1 shows the characteristics of the main scoring systems for use in CF.
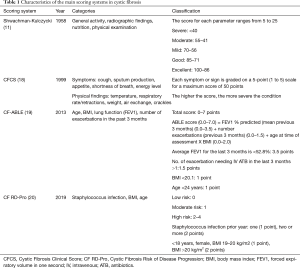
Full table
Overall, considering that CF is a progressive disease and continuous monitoring in order to early detect progression and quantify severity is greatly recommended, scoring systems may constitute an important tool to help (I) predicting the evolution of the disease, (II) establishing the rate of progression, (III) estimating the need for intervention, (IV) detecting therapeutic responses, and (V) selecting patients for special and immediate care. As there is no consensus over one particular score, the selection must consider the main purpose for its use in different clinical or research scenarios. It is also important to highlight that any chosen scoring system needs to reflect daily clinical practice and use objective clinical information in a standardized manner.
Acknowledgments
The authors would like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (finance code 001), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.121). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bradley JM, Moran FM, Stuart Elborn J. Evidence for physical therapies airway clearance and physical training in cystic fibrosis: An overview of five Cochrane systematic reviews. Respir Med 2006;100:191-201. [Crossref] [PubMed]
- Sturm R. An advanced stochastic model for mucociliary particle clearance in cystic fibrosis lungs. J Thorac Dis 2012;4:48-57. [PubMed]
- Castellani C, Cuppens H, Macek M Jr, et al. Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. Bone 2008;23:1-7. [PubMed]
- Nielsen KG, Pressler T, Klug B, et al. Serial Lung Function and Responsiveness in Cystic Fibrosis during Early Childhood. Am J Respir Crit Care Med 2004;169:1209-16. [Crossref] [PubMed]
- Sanders DB, Bittner RCL, Rosenfeld M, et al. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med 2010;182:627-32. [Crossref] [PubMed]
- Liou TG, Elkin EP, Pasta DJ, et al. Year-to-year changes in lung function in individuals with cystic fibrosis. J Cyst Fibros 2010;9:250-6. [Crossref] [PubMed]
- Heinzmann-Filho JP, Pinto LA, Marostica PJ, et al. Variation in lung function is associated with worse clinical outcomes in cystic fibrosis. J Bras Pneumol 2015;41:509-15. [Crossref] [PubMed]
- Cystic Fibrosis Foundation Patient Registry. 2015 Annual Data Report. Available online: Accessed Jan 20, 2020.https://www.cff.org/Our-Research/CF-PatientRegistry/2015-Patient-Registry-Annual-Data-Report.pdf
- Walshaw MJ. Cystic fibrosis: Diagnosis and management of - NICE guideline 78. Paediatr Respir Rev 2019;31:12-4. [Crossref] [PubMed]
- Santos CI da S. Critical analysis of scoring systems used in the assessment of cystic fibrosis severity: state of the art. J Barsileiro Pneumol 2004;30:286-98. [Crossref]
- Shwachman H, Kulczycki L. Long term study of one hundred five patients with cystic fibrosis. AMA J Dis Child 1958;96:6-15. [Crossref] [PubMed]
- Stollar F, Adde FV, Cunha MT, et al. Shwachman-Kulczycki score still useful to monitor cystic fibrosis severity. Clinics 2011;66:979-83. [Crossref] [PubMed]
- Chrispin AR, Norman AP. The systematic evaluation of the chest radiograph in cystic fibrosis. Pediatr Radiol 1974;2:101-5. [Crossref] [PubMed]
- Brasfield D, Hicks G, Soong S, et al. The Chest Roentgenogram in Cystic Fibrosis: A New Scoring System. Pediatrics 1979;63:24-9. [PubMed]
- Conway SP, Pond MN, Bowler I, et al. The chest radiograph in cystic fibrosis: A new scoring system compared with the Chrispin-Norman and Brasfield scores. Thorax 1994;49:860-2. [Crossref] [PubMed]
- Doershuk CF, Matthews LW, Tucker AS, et al. A 5 year clinical evaluation of a therapeutic program for patients with cystic fibrosis. J Pediatr 1964;65:677-93. [Crossref] [PubMed]
- Taussig LM, Kattwinkel J, Friedewald WT, et al. A new prognostic score and clinical evaluation system for cystic fibrosis. J Pediatr 1973;82:380-90. [Crossref] [PubMed]
- Kanga J, Kuhn R, Craigmyle L, et al. Cystic fibrosis clinical score: A new scoring system to evaluate acute pulmonary exacerbation. Clin Ther 1999;21:1343-56. [Crossref] [PubMed]
- McCarthy C, Dimitrov BD, Meurling IJ, et al. The CF-ABLE score: a novel clinical prediction rule for prognosis in patients with cystic fibrosis. Chest 2013;143:1358-64. [Crossref] [PubMed]
- Marsteller NL, Nussbaum E, Morphew T, et al. Cystic fibrosis patients at risk for disease progression marked by decline in FEV1% predicted: Development of the cystic fibrosis risk of disease progression score. J Thorac Dis 2019;11:5557-65. [Crossref] [PubMed]


