
Necessity of pleura repair in the chest wall reconstruction with three-dimensional printed titanium implant
The main indications for chest wall reconstruction include malignancies (primary or metastatic), infections, radiation-induced necrosis, trauma and herniation (1). There are few studies on the application of bioprosthetic materials in chest wall reconstruction. Bioprosthetic materials mainly include bovine pericardial patch, porcine mesenteric patch and artificial skin patch, which are less toxic and have no effect on cell activity (2,3). However, chest wall reconstruction using bioprosthetic materials often results in insufficient tensile strength to protect intra-thoracic organs and long-term deterioration of lung elasticity. Thus, it is important to reconstruct chest wall using rigid implants for extensive chest wall defects in clinical practice (4).
Three-dimensional printed (3DP) titanium implants have proved to be a novel and feasible material for individual chest wall reconstruction (5). However, titanium material has a strong hydrophobicity surface, which can prevent protein absorption and cell adhesion (6). Herein, we noticed that, the lung tissue cannot directly adhere to the 3DP titanium implants. Therefore, it is necessary to reconstruct the pleura on the inner surface of the implants. The cases are reported as follows.
Surgical technology
Five patients with chest wall lesion were retrospectively analyzed in Tangdu Hospital from July 2015 to June 2018. Table 1 shows the general characteristics and clinical features of 5 patients. All patients volunteered to participate in the study and signed informed consent forms. And all received a fine needle aspiration biopsy to obtain a pathologic diagnosis. Then positron emission tomography (PET), computed tomography (CT) and bone scintigraphic imaging was performed to identify the existence of distant metastasis in other organs. All of them received CT examination with slice thickness at 0.90 mm using a 64-detector CT scanner (GE LightSpeed VCT). Mimics (version 17.0, Materialise Inc., USA) and Geomagic Studio (version 2012, Geomagic, Inc., USA) software were used to make a surgical planning and implant designing. The prospective resection margin was estimated as a minimum distance of 3cm beyond the cancer. 3DP implants were designed and made simulating the same shape as the thoracic bony structure of the patient. Finally, the surgical-grade titanium alloy material was used to fabricate the implants in an EOS M280 3DP machine (EOS GmbH, Krailling, Germany).

Full table
All patients underwent wide excision of the lesion in the anterolateral or posterior chest wall, while the proximal pleura and muscles were removed. The disease-free resection margin was established during surgery on the basis of frozen tissue section analysis. An additional resection would be adopted if a positive margin was found. Then, the chest wall defect was reconstructed by individualized 3DP titanium implant, and the steel wire was used to bundle the prosthesis and residue ribs (Figure 1A). The myocutaneous pedicled flap was covered on the outer surface of the titanium implants. The pectoralis major myocutaneous flap and latissimus dorsi flap were used frequently.
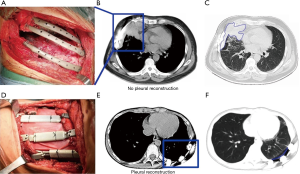
Pleural repair was implemented with the ThormalGEN surgical patch (Guanhao Biotech Corporate, Guangzhou, China) which was prepared from porcine tissues for all except case 1 and case 2. The basic structure of the patch remains identical while major component is collagen. Product is a flat sheet with a smooth side and a rough side. The patch was densely suspended on the inner surface of titanium implants and the rough side of the patch was in contact with the lung tissue. The edge of the patch was sutured continuously with the remaining pleura using silk or prolene suture on the pleural stump or intercostal muscle, and the patch was suspended on the prosthesis to make it in a tight state.
In the following period, chest CT images showed localized pleural effusion between lung and the titanium implants (Figure 1B,C). The patients were not extubated until the daily drainage is less than 40 mL in 2 months after surgery. For patients received pleural repair (Figure 1D), a negative pressure drainage tube was retained under the pedicled flap in surgery, and the drainage tube can be removed when daily drainage is less than 10 mL. The patients were discharged home 10 days after surgery and the chest CT scan showed a stable reconstruction (Figure 1E,F), preservation of thoracic morphology and excellent cosmetic results.
Discussion
Biomimetic reconstructions are expected to offer better function and cosmetic results after extensive chest wall resection. Therefore, the previous studies mostly focus on reconstructing bony structure of the chest wall (7,8). 3D computer-assisted design eliminates the need to shape, cut, or contour the implant and this procedure may produce a perfect anatomic fit. Moreover, the 3DP titanium implants can provide an almost infinite variety of designs of implants according to the individual demands of each patient, including rare or uncommon cases. In the development trend dominated by precision personalized medicine, the emergence of 3DP titanium implants will play a decisive role in the development of clinical treatment of chest wall surgery and gradually become the leading new technology in this field.
However, due to the hydrophobicity of the titanium, the lung tissue cannot adhere to the titanium implants directly. We underwent pleural repair with the ThormalGEN surgical patch. The major characteristics and functions of the patch are listed below: (I) provide isolation and reinforcement at damaged sites; (II) prevent tissues from leakage, rupture, scarring, and hernia; (III) exhibit good biocompatibility and histocompatibility; (IV) satisfy clinical requirements on mechanical properties in terms of anti-tensile strength, flexibility and suturability.
In this series, we evidenced that pleural repair is an essential key for chest reconstruction. Thanks to the good hydrophilicity and histocompatibility of ThormalGEN surgical patch, lung can adhere to it directly. The traumatic exudation after chest wall resection is mainly from the muscles and connective tissue around the wound. Once the pleura is reconstructed using the ThormalGEN surgical patch, the exudate can be effectively prevented from entering the pleural cavity. In addition, the closed thoracic drainage tube is not necessary for the patients with pleura reconstruction, with which procedure can helpfully and efficiently reduce postoperative patient pain and enhancing recovery. However, the ThormalGEN surgical patch is mechanically fixed to the implant by sutures, rather than firm biological connection, and the risk of loosening and detachment should be concerned in the future. In addition, the cost of biological patch is high, which increases the economic burden on patients. In the future, the bio-affinity of the titanium can be improved by surface modification to form strong structural connections.
Acknowledgments
Funding: This work was supported by Key Research and Development Plan in Shaanxi Province (2019SF-095, 2018SF-056), National Natural Science Foundation of China (51835010, 81901838), Key-Area Research and Development Program of Guangdong Province (2018B090906001).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.04.53). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All patients volunteered to participate in the study and signed informed consent forms.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Qin X, Tang H, Xu Z, et al. Chest wall reconstruction with two types of biodegradable polymer prostheses in dogs. Eur J Cardiothorac Surg 2008;34:870-4. [Crossref] [PubMed]
- Makarawo TP, Reynolds RA, Cullen ML. Polylactide bioabsorbable struts for chest wall reconstruction in a pediatric patient. Ann Thorac Surg 2015;99:689-91. [Crossref] [PubMed]
- Wiegmann B, Korossis S, Burgwitz K, et al. In vitro comparison of biological and synthetic materials for skeletal chest wall reconstruction. Ann Thorac Surg 2015;99:991-8. [Crossref] [PubMed]
- Weyant MJ, Bains MS, Venkatraman E, et al. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg 2006;81:279-85. [Crossref] [PubMed]
- Aranda JL, Jiménez MF, Rodríguez M, et al. Tridimensional titanium-printed custom-made prosthesis for sternocostal reconstruction. Eur J Cardiothorac Surg 2015;48:e92-4. [Crossref] [PubMed]
- Att W, Hori N, Iwasa F, et al. The effect of UV-photofunctionalization on the time-related bioactivity of titanium and chromium-cobalt alloys. Biomaterials 2009;30:4268-76. [Crossref] [PubMed]
- Mansour KA, Thourani VH, Losken A, et al. Chest wall resections and reconstruction: a 25-year experience. Ann Thorac Surg 2002;73:1720-5; discussion 1725-6.
- Wang L, Huang L, Li X, et al. Three-Dimensional Printing PEEK Implant: A Novel Choice for the Reconstruction of Chest Wall Defect. Ann Thorac Surg 2019;107:921-8. [Crossref] [PubMed]


