Subxiphoid completion thymectomy for refractory non-thymomatous myasthenia gravis
Introduction
Thymectomy is an effective surgical procedure complementing medical therapy of patients with myasthenia gravis (MG) (1,2). Extended thymectomy entailing complete removal of thymic and perithymic tissue has been advocated as the desired target for a fast and durable remission of MG symptoms (3). However, response to procedure is not often prompt and effective, ranging from 23.1% to 46% (4-6) in terms of complete remission rate at 5-year follow up. The presence of residual thymic tissue (7) or ectopic thymic tissue within the mediastinal fat tissue (8-11) still producing antibodies against the receptor for acetylcholine (anti-AChRAb) has been suggested as the main reason for post-thymectomy refractory MG. Indeed, despite the intent of radical thymectomy, residual thymic tissue can be present in 64–70% of thymectomized patients (7,11). In this regard, completion thymectomy has been advocated after unsuccessful thymectomy (12) with quite effective results by using extended either maximal trans-sternal (13,14) or, more recently, video-assisted transthoracic (VATS) approaches (15).
Lately, the subxiphoid route has been successfully proposed to achieve complete extended thymectomy yet avoiding trans-sternal as well as trans-costal routes (16). On these bases we applied the subxiphoid VATS in patients with non-thymomatous refractory MG to allow a complete and definitive clearance from residual thymic tissue scattered within the thymic bed and elsewhere in the mediastinum or in lower neck. Hereby we present our short- and long-term results using this approach to accomplish completion thymectomy.
Methods
Between July 2010 and December 2017, 15 consecutive patients with refractory non-thymomatous MG, 8 women and 7 men with a median age of 44 years and an interquartile range (IQR) of 38.5 and 53.5 years (range, 31–59), underwent video-assisted completion thymectomy through a subxiphoid approach.
Study design
This is a retrospective observational study focused on simple evaluation of rare cases operated through a peculiar approach for an uncommon pathologic condition that is non-thymomatous MG refractory to primary surgery. Due to the rarity of the condition and the retrospective nature of the study, our Internal Review Board exempted us from asking patient’s permission.
Study group
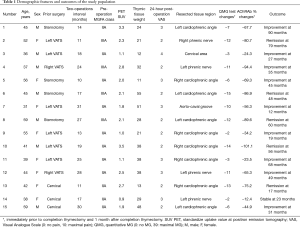
In the study group we included all patients undergoing subxiphoid completion thymectomy after a previous intentional total-thymectomy for non thymomatous MG. Patients refractory to thymectomy were considered those with worsening as well as those experiencing unmodified persistence of the initial preoperative myasthenic symptoms for more than one year postoperatively. Mandatory prerequisite for completion thymectomy was the evidence of macroscopic residual thymus and/or mediastinal fat tissue, preferably sited in the lower regions of the mediastinum (i.e., cardio-phremic angle, beyond phrenic nerve), demonstrable at computed tomography (CT) and/or positron emission tomography (PET) or magnetic resonance. Clinical data of the study group is summarized in Table 1. The median duration of MG before the initial operation was 21 months (IQR, 15–29 months and range, 12–38). The prior operations had been performed in our institution in 7 (46%) cases, where VATS thymectomy was performed in 4 patients and transcervical thymectomy was performed in 3 cases. The remaining 8 patients came from other institutions, 4 after VATS and 4 after transsternal thymectomy. The median interval between initial thymectomy and completion procedure was 18 (IQR, 13.5–26) months, ranging from 10 to 31 months.
Full table
Preoperative study
The worsening as well as the unmodified persistence of the initial preoperative myasthenic symptoms after thymectomy for more than 1 year defines the status of refractory MG. In these patients we repeated all our routine diagnostic workup including imaging, instrumental and laboratory reassessment as if the patient had never been worked-up before. CT scan was always performed, preferably associated to PET and/or magnetic resonance.
All patients presented characteristic decreasing responses to low frequency repetitive nerve stimulation. Thirteen patients had elevated serum titers of anti-AChRAb (Table 1).
Decision of carrying out a completion thymectomy was always made after a panel discussion with neurologists, anesthesiologists, intensivists and physiotherapists. Fully informed consent was obtained from all patients, who were made thoroughly aware of the benefit and risks of the subxiphoid approach including all the possible complications.
Preoperative stabilization
At admission, every patient presented a class 2 or 3 MG (Table 1) without bulbar symptoms. They were all receiving large doses of anticholinesterases [pyridostigmine bromide, median 560 mg/day (range, 330–740 mg/day)] associated with steroids [prednisone, median 45 mg/day (range, 30–58 mg/day)]. All efforts to decrease medications were unsuccessful because of increased fatigue. Eight patients underwent plasma exchange immediately before surgery.
Surgery
Nasogastric tube was always inserted to allow early-postoperative administration of anticholinesterase drugs. Double-lumen intubation was also deemed mandatory in all patients.
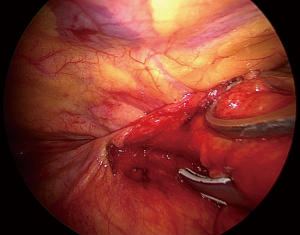
A 2.5–3.5 cm transverse skin incision was made 1–2 cm below the lower edge of the xiphoid (Figure 1). The anterior layer of the rectus sheath was cut and digitally dissected on the reverse side of the xiphoid. A plastic self-standing sleeve retractor (Alexis® Applied Medical, Amersfoort, Netherlands) was inserted and fixed. Supplementary transthoracic port may be added to facilitate lateral vision and dissection along the phrenic nerve.
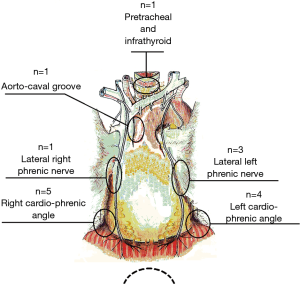
The procedure routinely included en bloc removal of the residual thymus, if present, or all of the mediastinal lower cervical perithymic adipose tissue (Figure 2). The thymus bed was carefully inspected and any residual tissue suspected at preoperative imaging was specifically searched. Once identified, the residual thymic tissue was carefully dissected avoiding prolonged manipulation and capsule tears. Subsequently, a systematic resection of all mediastinal fat was accomplished including retro-innominate area, aorto-caval groove, aorto-pulmonary window, cervical area and pro-pericardium region. Furthermore, both pleural spaces were routinely opened, and cardio-phrenic angle areas were dissected bilaterally. Ultimately the fat beyond the phrenic nerve was also carefully removed. All these steps were necessary in order to achieve the best results from the procedure. All resected specimens were separately removed using an endobag through the subxiphoid route and disposed on a dedicated paper map to facilitate pathologist’s orientation.
Postoperative evaluation
The therapeutic effect of completion thymectomy was investigated for each patient by comparing the preoperative clinical status with the status at the most recent follow-up examination.
Objective physical examination was based on the quantitative MG test, which explores skeletal muscle function in the limbs, head, and neck as well as respiratory, swallowing, and speaking capabilities (17,18). For the initial evaluation, patients were preferably required to be off anticholinesterases for 12 hours before testing. The final score included the sum of 13 tests after which the examiner could assign a partial score from 0 (absent) to 3 (maximum) so that the total ranged from 0 to 39. Quantitative MG test, electromyography and anti-AChRAb dosages were performed at one month postoperatively and matched with preoperative values.
Statistical analysis
Statistical analysis was performed using the SPSS package, version 20.0 (IBM, Armonk, NY, USA). Descriptive statistics was presented as median and interquartile range. Due to the non-normal distribution of some variables, non-parametric tests for paired, and unpaired comparisons (Wilcoxon-sum rank and Mann-Whitney, respectively) were used. Significance was set at P<0.05.
Complete stable remission was defined for those patients asymptomatic and off-medications (except for 10 mg/day or less of methylprednisolone) for at least 12 months. Time to complete stable remission from thymectomy was estimated with the Kaplan-Meier method. Patients in no-remission were censored and their time to complete stable remission was defined as time from operation to most recent patient follow-up.
Results
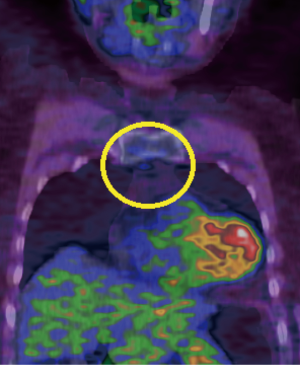
In 11 cases, areas of residual active thymus had been identified as mildly avid regions [standardized uptake value (SUV) more than or equal to 1.8) at PET compared to normal mediastinal background activity (Figure 3) (Table 1). In the other 4 cases, we had noticed only residual fat tissue deposits at CT in the mediastinal area. Median operative time was 106 (IQR, 77–141) minutes, ranging from 90 to 190 minutes. Median weight of the removed tissue was 29 (IQR, 21–38) grams (Table 1). Thymic residuals were found and histologically proved in all cases.
Intraoperative findings
No thymomatous transformation was found in any patient. Table 1 enumerates the site of residual thymic tissue in relation to the previous approach. Interestingly, we found that the most frequent site of ectopic thymus after median sternotomy was the left cardiophrenic angle beyond the phrenic nerve. After unilateral VATS, thymic remnants were mainly found in the contralateral cardiophrenic angle (n=4).
Postoperative recovery
No operative deaths nor major morbidity occurred. One-day postoperative Visual Analogue Scale value was 2.53±0.63. Median hospital stay was 2 (IQR, 1–3.5) days (range, 1–9 days). All patients returned to normal activities within 2 weeks after operation, and 80% were satisfied with their treatment. Only one patient required further plasma exchange treatment because of a myasthenic crisis, which occurred after 3 months. We experienced a significant decrease of the anti-AChRAb at one month [median percentage changes −67% (IQR, −39% to −83%)].
Long-term results
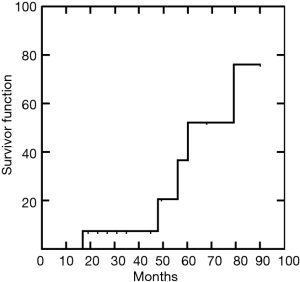
Median follow-up was 45 (IQR, 21–58) months (range, 12–90). At the most recent follow-up complete stable remission was achieved in 5 patients (Figure 4). Another 9 patients had significant improvement in bulbar and limb function, requiring lower doses of corticosteroids and anticholinesterase drugs. Only one patient remained clinically stable although drugs dosage was reduced. Interestingly, we found the one-month postoperative drop of anti-AChRAb greater than median value was significantly correlated with complete stable remission after completion thymectomy (P=0.002).
Discussion
Completion thymectomy demonstrated a clear effectiveness in treating those patients who develop MG refractory to initial thymectomy (12,13). In a recent best evidence study Ng et al. showed that repeat thymectomy may improve myasthenic symptoms in 60–70% of the patients and especially in those who firstly undergo trans-cervical thymectomy (19). This effect likely depends on the difficulty in reaching complete extended thymectomy through the trans-cervical approach (13,14). Nevertheless, despite the radical intent, we also experienced refractory MG after unilateral VATS (15), especially when this procedure had been carried out by our residents. This event is attributable to the persistence of contralateral thymic remnants or perithymic fat tissue, which represents one of the pitfalls of the unilateral trans-pleural procedure. However, we described refractory MG even after partial trans-sternal thymectomy (15). In this case MG was mostly sustained by ectopic thymic tissue located in the left pericardio-phrenic angle, which may be tricky to resect through an antero-superior access.
Over the time, many options have been proposed to increase efficacy and tolerance of the repeated procedure (20). First of all, it is recommended to avoid a redo-sternotomy especially in these patients who are often under chronic steroid-based pharmacological regimen, thus increasing the risk of sternal wound infections (15). Second, the procedure should be attempted through a different approach in order to avoid the possible presence of postoperative adhesions (20). Third, the completion procedure should take into account the site of the supposed residual thymic tissue (8). For this reason, it is pivotal to pursue an accurate imaging evaluation demonstrating the presence of potential residual thymic or fat tissue and determining their metabolic activity. We have already described the diagnostic yield of PET/CT in the assessment of the activity of thymic and perithymic tissue (21). Hereby we documented the importance of PET/CT in ameliorating the effects of completion thymectomy. In all described cases we have intentionally performed the resection of a targeted area, which always resulted residual or ectopic thymic tissue at histological examination.
In order to fulfill these requirements up to 2010 we used to carry out completion thymectomy through unilateral VATS approach (15). Since that time, with the confidence acquired by routine use of the subxiphoid approach in many pathologic conditions, we started to exploit this route for repeated thymectomy. We did not find difficulties in employing this access even when previous sternotomy had been performed. Incidental adhesions are usually weak and rarely interfere on visualization. Subxiphoid approach allows marvellous exposure of the whole mediastinal field making possible a complete thymic tissue clearance and mediastinal fat tissue exenteratio. The introduction of curved and articulating instruments allows to reach tissue beyond the phrenic nerve and in the cervical area.
We perfectly acknowledge that the study has intrinsic limitations of the absence of a control group and the wide timespan. All these flaws are inevitable due to the rarity of the condition, but they are partially mitigated by the homogeneity of data provided by a substantially unchanged MG Unit throughout this long period. On the other hand, medical therapy for MG has not remarkably changed during the last twenty years. On the bases of our results we believe that the subxiphoid approach might be considered a reliable approach for repeat thymectomy. It allowed to reach any district where residual or ectopic thymic tissue might be potentially hidden. It is better accepted by both patients and neurologists because it is one single, limited and almost painless incision. In our experience clinical improvement clearly correlated with the reoperation and namely with the drop of circulating antibodies.
Conclusions
In conclusion this initial experience confirms that repeat thymectomy is a useful procedure in refractory MG. The removal of ectopic and residual thymus through a subxiphoid approach can reduce anti-AChRAb titer. This event is correlated to positive outcome of refractory MG.
Acknowledgments
During the editing of this paper Professor Tommaso Claudio Mineo, who inspired and initiated this study, sadly died. The authors feel very grateful and deeply in debt to him.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.81). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized trial of thymectomy in myasthenia gravis. N Engl J Med 2016;375:511-22. [Crossref] [PubMed]
- Yang J, Liu C, Li T, et al. Prognosis of thymectomy in myasthenia gravis patients with thymus hyperplasia. Int J Neurosci 2017;127:785-9. [Crossref] [PubMed]
- Jaretzki A 3rd, Penn AS, Younger DS, et al. “Maximal” thymectomy for myasthenia gravis:results. J Thorac Cardiovasc Surg 1988;95:747-57. [Crossref] [PubMed]
- Mack MJ, Landreneau RJ, Yim AP, et al. Results of video-assisted thymectomy in patients with myasthenia gravis. J Thorac Cardiovasc Surg 1996;112:1352-60. [Crossref] [PubMed]
- Nakamura H, Taniguchi Y, Suzuki Y, et al. Delayed remission after thymectomy for myasthenia gravis of the purely ocular type. J Thorac Cardiovasc Surg 1996;112:371-5. [Crossref] [PubMed]
- Masaoka A, Yamakawa Y, Niwa H, et al. Extended thymectomy for myasthenia gravis patients: a 20-year review. Ann Thorac Surg 1996;62:853-9. [Crossref] [PubMed]
- Rosenberg M, Jáuregui WO, De Vega ME, et al. Recurrence of thymic hyperplasia after thymectomy in myasthenia gravis. Its importance as a cause of failure of surgical treatment. Am J Med 1983;74:78-82. [Crossref] [PubMed]
- Masaoka A, Nagaoka Y, Kotake Y. Distribution of thymic tissue at the anterior mediastinum. Current procedures in thymectomy. J Thorac Cardiovasc Surg 1975;70:747-54. [Crossref] [PubMed]
- Fukai I, Funato Y, Mizuno T, et al. Distribution of thymic tissue in the mediastinal adipose tissue. J Thorac Cardiovasc Surg 1991;101:1099-102. [Crossref] [PubMed]
- Ashour M. Prevalence of ectopic thymic tissue in myasthenia gravis and its clinical significance. J Thorac Cardiovasc Surg 1995;109:632-5. [Crossref] [PubMed]
- Ambrogi V, Mineo TC. Active ectopic thymus predicts poor outcome after thymectomy in class III myasthenia gravis. J Thorac Cardiovasc Surg 2012;143:601-6. [Crossref] [PubMed]
- Miller RG, Filler-Katz A, Kiprov D, et al. Repeat thymectomy in chronic refractory myasthenia gravis. Neurology 1991;41:923-4. [Crossref] [PubMed]
- Masaoka A, Monden Y, Seike Y, et al. Reoperation after transcervical thymectomy for myasthenia gravis. Neurology 1982;32:83-5. [Crossref] [PubMed]
- Henze A, Biberfeld P, Christensson B, et al. Failing transcervical thymectomy in myasthenia gravis. An evaluation of transsternal re-exploration. Scand J Thorac Cardiovasc Surg 1984;18:235-8. [Crossref] [PubMed]
- Mineo TC, Pompeo E, Ambrogi V, et al. Video-assisted completion thymectomy in refractory myasthenia gravis. J Thorac Cardiovasc Surg 1998;115:252-4. [Crossref] [PubMed]
- Suda T, Sugimura H, Tochii D, et al. Single-port thymectomy through an infrasternal approach. Ann Thorac Surg 2012;93:334-6. [Crossref] [PubMed]
- Barohn RJ, McIntire D, Herbelin L, et al. Reliability testing of the quantitative myasthenia gravis score. Ann NY Acad Sci 1998;841:769-72. [Crossref] [PubMed]
- Barnett C, Katzberg H, Nabavi M, et al. The quantitative myasthenia gravis score: comparison with clinical, electrophysiological, and laboratory markers. J Clin Neuromuscul Dis 2012;13:201-5. [Crossref] [PubMed]
- Ng JK, Ng CS, Underwood MJ, et al. Does repeat thymectomy improve symptoms in patients with refractory myasthenia gravis? Interact Cardiovasc Thorac Surg 2014;18:376-80. [Crossref] [PubMed]
- Zieliński M, Kuzdzał J, Staniec B, et al. Extended re-thymectomy in the treatment of refractory myasthenia gravis: original video-assisted technique of re-sternotomy and results of the treatment in 21 patients. Interact CardioVasc Thorac Surg 2004;3:376-80. [Crossref] [PubMed]
- Mineo TC, Ambrogi V, Schillaci O. May positron emission tomography reveal ectopic or active thymus in preoperative evaluation of non-thymomatous myasthenia gravis? J Cardiothorac Surg 2014;9:146. [Crossref] [PubMed]